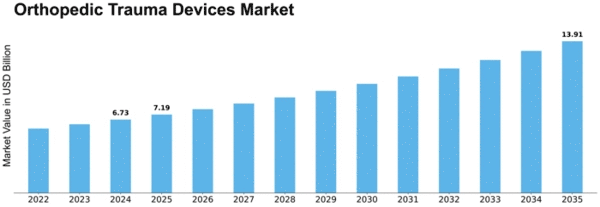
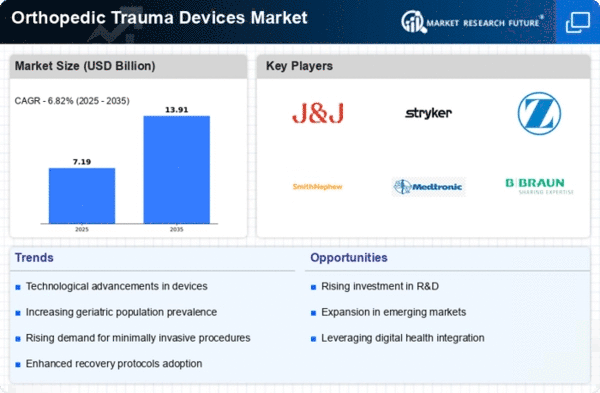
Orthopedic Trauma Devices Size
Orthopedic Trauma Devices Market Growth Projections and Opportunities
The market is impacted by various factors that drive its growth and development. One of the main causes is increased musculoskeletal injuries and fractures due to accident-related events, sports and an aging population among others. The world has a growing number of old people therefore more often they have fractured bones as well as orthopedic trauma increasing demand for trauma devices globally which help in surgical treatment stabilizing fracture thus aid patient recovery. Another significant determinant is modern medical technology. Continued innovations in materials, design and manufacturing techniques have produced more sophisticated and efficient orthopedic trauma devices. For example, implants made up of biocompatible materials like stainless steel and titanium provide better stability besides reducing risks involved with complications arising from them after implantation. Furthermore, government regulations shape the Orthopedic Trauma Devices Market significantly. Various regulatory agencies such as U.S Food & Drug Administration (FDA) or European Medicines Agency (EMA) set strict rules to ensure safety and efficacy of orthopedic devices. Players must ensure their products meet these standards according to quality controls before selling them locally or international markets through seeking approvals from bodies such as FDA otherwise they can be sued by customers or banned from operating in those markets.
Health infrastructure development worldwide underpins this market factor principally. Globalization necessitates improving healthcare facilities thus advanced medical technologies like orthopedic trauma devices need to be adopted in these scenarios too. The emerging economies are witnessing a high demand for orthopedic trauma devices because the population in these countries is increasingly becoming aware of the significance of timely and effective orthopedic interventions.
The Orthopedic Trauma Devices Market in turn is also affected by the growing trend towards personalized medicine and patient specific implants.Currently, manufacturers are concentrating more on providing customized solutions that can fit individual patients’ unique anatomical characteristics. Consequently, this results to an improvement in effectiveness of orthopedic treatments generally besides reducing risks which are associated with other complications thus improving patient results.
Economic factors like healthcare spending on products and services and insurance-reimbursement policies have considerable impacts on market dynamics.In addition, accessibility and affordability of these devices especially trauma ones are important determinant for their uptake.Market players are trying to come up with inexpensive alternatives devoid of compromising quality so that their use is broadened to at least reach a larger group among patients.
Collaborations between medical device manufacturers, research institutions, and healthcare providers contribute significantly to market expansion.This means less time taken by doctors while carrying out research hence leading faster innovations regarding orthopedic trauma devices that could be introduced into the market.The reason behind joint ventures is for firms to share expertise in order to come up with cutting edge technologies offered as well as competitive advantage.

















Leave a Comment