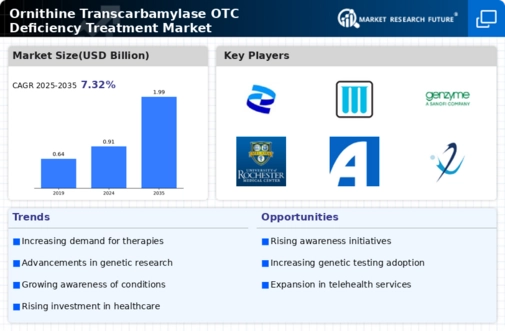
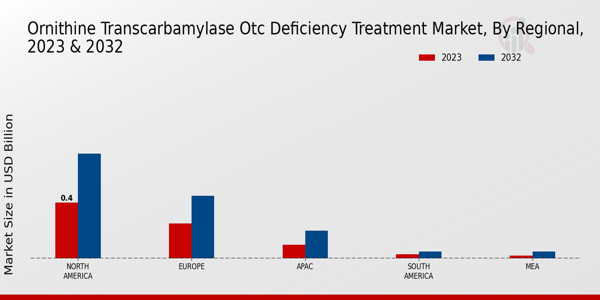
Market Growth Projections
The Global Ornithine Transcarbamylase OTC Deficiency Treatment Market Industry is projected to experience substantial growth over the next decade. With a market value of 0.91 USD Billion in 2024, it is expected to reach 1.99 USD Billion by 2035, reflecting a compound annual growth rate (CAGR) of 7.35% from 2025 to 2035. This growth trajectory is indicative of the increasing demand for effective treatments and the ongoing advancements in medical research. Factors such as rising prevalence rates, regulatory support, and innovations in treatment options are likely to contribute to this upward trend, positioning the market for significant expansion in the coming years.
Advancements in Treatment Options
Innovations in treatment methodologies are significantly influencing the Global Ornithine Transcarbamylase OTC Deficiency Treatment Market Industry. Recent developments in enzyme replacement therapies and gene therapy offer new avenues for managing this condition. These advancements not only improve patient outcomes but also enhance the quality of life for individuals affected by OTC deficiency. The introduction of novel therapies is anticipated to attract investment and research, further stimulating market growth. As these treatments become more widely available, the market is projected to expand, potentially reaching 1.99 USD Billion by 2035, reflecting a robust CAGR of 7.35% from 2025 to 2035.
Regulatory Support and Incentives
Regulatory frameworks and incentives play a crucial role in shaping the Global Ornithine Transcarbamylase OTC Deficiency Treatment Market Industry. Governments and health authorities are increasingly recognizing the need for effective treatments for rare genetic disorders. Initiatives such as orphan drug designations and funding for research and development are likely to encourage pharmaceutical companies to invest in OTC deficiency treatments. This supportive environment can lead to accelerated approval processes for new therapies, thereby enhancing market growth. As regulatory bodies continue to prioritize rare diseases, the market is expected to benefit from increased innovation and a broader range of treatment options.
Increasing Prevalence of OTC Deficiency
The rising incidence of Ornithine Transcarbamylase OTC deficiency is a primary driver for the Global Ornithine Transcarbamylase OTC Deficiency Treatment Market Industry. As awareness of genetic metabolic disorders grows, more cases are being diagnosed. In 2024, the market is valued at approximately 0.91 USD Billion, reflecting the urgent need for effective treatments. The increasing number of newborn screenings and genetic testing initiatives contributes to this trend, as early diagnosis allows for timely intervention. This heightened awareness and diagnosis are expected to propel the market forward, indicating a growing demand for innovative therapies and management strategies.
Market Dynamics and Competitive Landscape
The competitive landscape of the Global Ornithine Transcarbamylase OTC Deficiency Treatment Market Industry is characterized by a dynamic interplay of established pharmaceutical companies and emerging biotech firms. This competition fosters innovation and drives the development of new therapies. Companies are increasingly focusing on strategic partnerships and collaborations to enhance their product offerings and expand their market reach. The presence of multiple players in the market is likely to lead to competitive pricing and improved access to treatments for patients. As the market evolves, the competitive dynamics will continue to shape the landscape, influencing treatment availability and patient outcomes.
Growing Awareness and Education Initiatives
Increased awareness and educational initiatives surrounding Ornithine Transcarbamylase OTC deficiency are pivotal for the Global Ornithine Transcarbamylase OTC Deficiency Treatment Market Industry. Organizations and healthcare providers are actively promoting knowledge about the disorder, its symptoms, and the importance of early diagnosis. This educational push is crucial in reducing the stigma associated with genetic disorders and encouraging families to seek testing and treatment. As awareness grows, more patients are likely to pursue treatment options, thereby driving market demand. This trend is expected to contribute to the overall expansion of the market, fostering a more informed patient population.





















Leave a Comment