Rising Geriatric Population
The increasing geriatric population is a significant factor driving The Global Oral Cancer Treatment Industry. As the global population ages, the incidence of oral cancer is expected to rise, given that older adults are more susceptible to various health conditions, including cancer. By 2030, it is projected that the number of individuals aged 65 and older will reach approximately 1.4 billion, creating a larger demographic at risk for oral cancer. This demographic shift necessitates the development of tailored treatment options that cater to the unique needs of older patients. Consequently, pharmaceutical companies and healthcare providers are likely to focus on creating therapies that are not only effective but also consider the comorbidities often present in this age group. This trend is expected to significantly influence market growth.
Growing Awareness and Education
The increasing awareness and education regarding oral cancer are pivotal drivers for The Global Oral Cancer Treatment Industry. Public health campaigns and educational initiatives have been instrumental in informing individuals about the risks associated with oral cancer, leading to earlier diagnosis and treatment. This heightened awareness is reflected in the rising number of screenings and consultations, which have surged by approximately 20% in recent years. As more individuals recognize the importance of oral health, the demand for effective treatment options is expected to rise correspondingly. Additionally, healthcare providers are focusing on educating patients about available therapies, which may further enhance treatment adherence and outcomes. This trend underscores the critical role of education in shaping market dynamics.
Government Initiatives and Funding
Government initiatives and funding aimed at combating oral cancer are crucial drivers for The Global Oral Cancer Treatment Industry. Various governments are implementing policies to enhance cancer care, including increased funding for research and development of new treatment options. For instance, several countries have established national cancer control programs that prioritize oral cancer, leading to improved access to treatment and care. This support is vital for fostering innovation within the industry, as it encourages collaboration between public and private sectors. Additionally, government-sponsored awareness campaigns are helping to educate the public about oral cancer prevention and treatment, further driving demand for effective therapies. As these initiatives continue to expand, they are likely to have a lasting impact on the market.
Increasing Incidence of Oral Cancer
The rising incidence of oral cancer is a primary driver for The Global Oral Cancer Treatment Industry. According to recent statistics, oral cancer cases have been steadily increasing, with an estimated 377,000 new cases reported annually. This alarming trend is attributed to various factors, including tobacco use, alcohol consumption, and the human papillomavirus (HPV). As awareness of these risk factors grows, healthcare systems are compelled to enhance their treatment offerings. Consequently, pharmaceutical companies are investing in research and development to create innovative therapies, thereby expanding the market. The increasing prevalence of oral cancer not only necessitates advanced treatment options but also drives demand for early detection and preventive measures, further propelling the market forward.
Advancements in Treatment Modalities
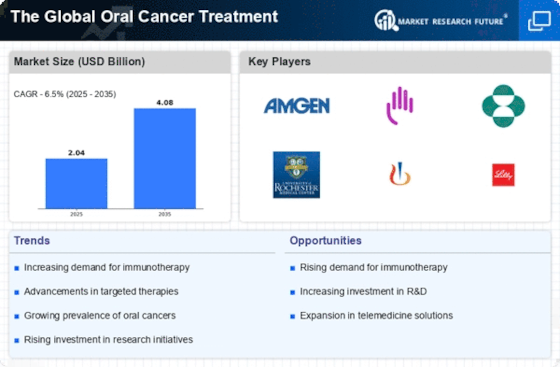
Technological advancements in treatment modalities are significantly influencing The Global Oral Cancer Treatment Industry. Innovations such as targeted therapies, immunotherapy, and minimally invasive surgical techniques are transforming the landscape of oral cancer treatment. For instance, targeted therapies have shown promising results in improving patient outcomes, leading to a projected market growth rate of approximately 7.5% over the next five years. These advancements not only enhance the efficacy of treatments but also reduce side effects, making them more appealing to patients. Furthermore, the integration of artificial intelligence in treatment planning and patient management is expected to streamline processes and improve overall care. As these technologies continue to evolve, they are likely to attract more investments, thereby fostering growth in the market.