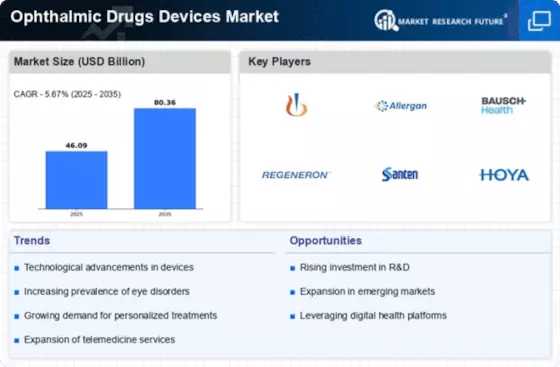
Top Industry Leaders in the Ophthalmic Drugs Devices Market

Latest Ophthalmic Drugs and Devices Companies Update:
US FDA approves generic Loteprednol Etabonate Drops (Lupin): On December 27, 2023, Indian drugmaker Lupin announced FDA approval of their generic equivalent of Bausch + Lomb's loteprednol etabonate ophthalmic suspension 0.2% for seasonal allergies. This approval opens up potential cost-effective options for patients.
Nordic Pharma acquires Visant Medical and launches Lacrifill for Dry Eye in US: On December 18, 2023, Nordic Pharma subsidiary Amring Pharmaceuticals acquired Visant Medical and announced plans to launch its Lacrifill cross-linking device for dry eye treatment in the US. This minimally invasive procedure offers an alternative to chronic eye drops for some patients.
Santen and Veriforce partner on AI-powered retinal disease detection platform: In January 2024, Japanese pharmaceutical company Santen announced a partnership with Veriforce for its AI platform EyeQ AI which assists in earlier diagnosis and management of retinal diseases. Such collaborations accelerate innovation in diagnostic tools.
J&J Vision and Alcon sign co-development and commercialization agreement for presbyopia treatment: On January 10, 2024, Johnson & Johnson Vision and Alcon announced a collaboration to develop and commercialize a novel treatment for presbyopia, a common age-related vision condition. This joint effort combines expertise and resources to bring potentially faster solutions to market.
List of Ophthalmic Drugs and Devices Key companies in the market
- Allergan plc (Ireland)
- Johnson & Johnson Vision (US)
- Alcon (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Ocular Therapeutix, Inc. (US)
- Lomb Incorporated (Canada)
- Sight Sciences, Inc. (US)
- Mibo Medical Group (US)
- BioTissue (US)
- NuSight Medical (US).