Market Share
Neuroblastoma Market Share Analysis
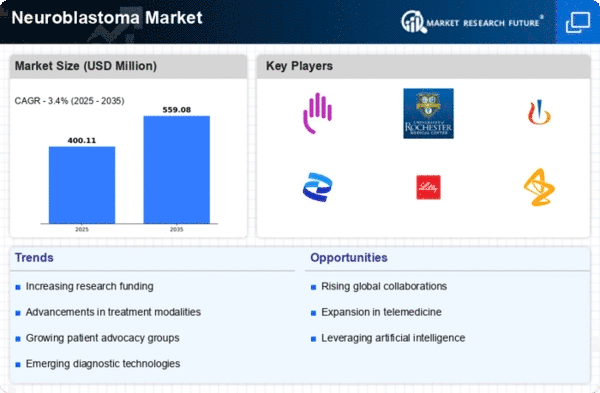
In the intricate landscape of the Neuroblastoma Market, companies employ various strategies to secure a competitive foothold and advance their market share. One primary approach involves differentiation, wherein companies focus on developing innovative and targeted therapies for neuroblastoma treatment. This might include novel drugs, immunotherapies, or precision medicine tailored to specific genetic markers associated with the disease. By offering unique and effective treatment options, companies can distinguish themselves in the market, attracting the attention of healthcare professionals, patients, and caregivers, and ultimately securing a distinct market share.
Cost leadership is a pivotal strategy in the Neuroblastoma Market, emphasizing the delivery of cost-effective therapies without compromising on quality. Achieving economies of scale through efficient manufacturing processes, strategic partnerships, and optimized distribution allows companies to offer competitive pricing. This cost-effective positioning appeals to a broad range of healthcare providers and can be particularly crucial in gaining market access, positioning the company as a reliable and affordable choice for neuroblastoma treatment.
Market segmentation plays a crucial role in market share positioning strategies for neuroblastoma therapies. Companies identify specific patient demographics, disease stages, or genetic subtypes and tailor their treatments to meet the unique needs of these segments. Whether focusing on high-risk patients, relapsed cases, or those with specific genetic mutations, segmentation enables companies to effectively address the diverse requirements of different patient groups. This targeted strategy can result in increased market share within the selected segments.
Strategic collaborations and partnerships are instrumental in expanding market reach and enhancing positioning in the Neuroblastoma Market. Collaborating with research institutions, oncology centers, or patient advocacy groups can lead to increased credibility, access to clinical trial networks, and a broader patient base. Such alliances may also facilitate joint research efforts, fostering innovation within the industry and contributing to a stronger market position.
Embracing advancements in precision medicine and targeted therapies is becoming increasingly crucial for market share positioning in the Neuroblastoma Market. Companies that invest in understanding the molecular and genetic intricacies of neuroblastoma and develop therapies tailored to specific patient profiles can offer more effective and personalized treatment options. Embracing these scientific advancements not only improves patient outcomes but also positions companies as leaders in providing cutting-edge solutions for neuroblastoma.
Customer relationship management (CRM) is fundamental for market share positioning in the Neuroblastoma Market. Establishing strong relationships with oncologists, pediatricians, and patient support groups, ensuring effective communication, and providing comprehensive support contribute to customer satisfaction and loyalty. A satisfied healthcare professional or patient is more likely to choose a specific therapy, fostering brand loyalty and potentially expanding market share through positive word-of-mouth referrals.
Adapting to regulatory changes and navigating the complex landscape of drug development regulations is an imperative strategy for companies operating in the Neuroblastoma Market. Staying compliant with evolving regulatory standards ensures the safety and efficacy of neuroblastoma therapies. Proactive engagement with regulatory bodies, adherence to ethical standards, and investment in research and development are crucial components of this strategy. By demonstrating a commitment to compliance and patient safety, companies can enhance their reputation and solidify their market position.

















Leave a Comment