Market Analysis
In-depth Analysis of Multiple Myeloma Treatment Market Industry Landscape
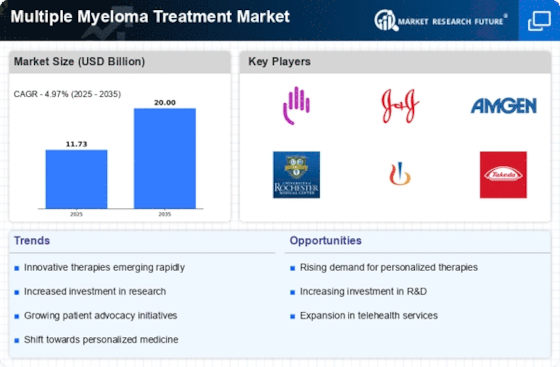
The multiple myeloma treatment market is driven by pharmaceutical advancements, continuous research, and rising prevalence. Technological innovation drives the multiple myeloma therapy market. Novel targeted medicines, immunomodulatory medications, and monoclonal antibodies have greatly improved multiple myeloma therapy choices, making them more tailored and effective. These developments attempt to improve patient outcomes, lengthen survival, and reduce hematological malignancy challenges.
Multiple myeloma, a plasma cell malignancy, is on the rise, driving the therapy industry. Advanced and comprehensive multiple myeloma treatment techniques are needed as global prevalence climbs. Aging populations and better diagnostics boost multiple myeloma detection, spurring research and development of novel treatments.
Multiple myeloma therapy market trends depend on regulation. New medications and treatment methods must meet strict safety and effectiveness requirements before being approved and sold. Compliance with regulatory regulations is crucial for market approval, patient and healthcare professional confidence, and innovative therapy development. The regulatory environment affects medication approvals, clinical trial design, and novel therapy market introduction.
Economic factors and healthcare reimbursement policies affect market dynamics. The cost-effectiveness of multiple myeloma treatments, reimbursement rules, and economic burden affect their acceptance by healthcare providers and institutions. Access to successful multiple myeloma treatments depends on the price and accessibility of innovative medications and future therapeutic techniques.
Competition among leading market participants drives multiple myeloma treatment market dynamics. Treatment efficacy, safety, mechanisms of action, and patient suitability help pharmaceutical businesses differentiate their medications. Research and development emphasis on combination medicines, resistance management, and novel intervention targets. Strategic partnerships with academic institutions, clinical trial networks, and advocacy groups boost market presence and innovation.
COVID-19 has affected patient care, clinical trial timeframes, and therapy access in the multiple myeloma treatment market. The epidemic highlighted the necessity of telemedicine, remote monitoring, and patient support in multiple myeloma management. The epidemic sparked clinical trial contingency planning and expedited digital health technology adoption in multiple myeloma care.

















Leave a Comment