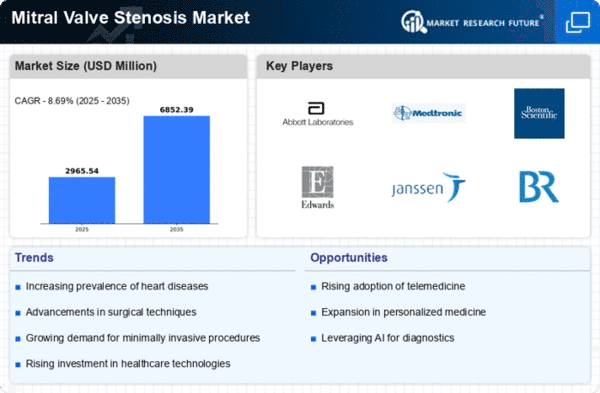
Market Analysis
In-depth Analysis of Mitral Valve Stenosis Market Industry Landscape
Mitral valve stenosis (MVS), a disease state in which the mitral valve in the heart becomes narrow, is one of the conditions that is still commonly seen in clinical practice with the growing demand for treatment getting better. The market dynamics for point-of-care solutions in mitral valve stenosis are driven by diverse influences consisting of the technological improvements, healthcare infrastructure as well as the continually rising awareness of both healthcare providers and patients.
Technological advancement and changing medical devices are crucial factors influencing the dynamics. Progress in diagnosis tools and treatment methodology has facilitated the application of more precise and effective point-of-care techniques for mitral valve stenosis. Technologies such as portable echocardiography devices and point-of-care ultrasound machines now exist which facilitate quicker and more accessible diagnosis which get to the bottom of a patient’s problem quickly and improve patient outcomes. Therefore, these technical innovations not only increase the efficiency of medical delivery mechanisms but also support the general development of mitral valve stenosis market.
The health care infrastructure is a key component in addressing the availability of mitral valve points of care for people with stenosis of the valve. Those regions which already have a developed healthcare environment will probably be able to use these solutions and incorporate them into the routine clinical practice much more successfully. The penetration into the market of developed countries increases due to their quality of infrastructure which is advanced, while emerging economies have resource availability and skill problems in the healthcare professionals’ sector. Therefore, the market dynamics are essentially dependent upon the health disparities of facilities between different regions.
The significant part of the market dynamics has been the growth of knowledge among health care providers and patients regarding mitral valve stenosis. To keep up with expanding medical knowledge and momentum in health awareness campaigns, there is a gradual shift to detecting cardiovascular conditions including mitral valve stenosis at early stages. It does not only cause the demand for point-of-care solutions but also pushes research and ploughing into technological innovations such as improvement of current tech and development of new more efficient diagonistic and therapeutic technologies.
Besides that, much of the pattern of market growth is shaped by the surrounding regulatory environment for the point-of-care solutions aimed at stenosis of mitral valve. Following strict regulatory procedures and approval protocols that guarantee the effectiveness and security for these medical devices, the confidence of both doctors and patients is restored. Regulation is one of the essential factors which determine the entry and competition of manufacturers, and thus regulates the entire mitral valve stenosis market stability and reliability.
















Leave a Comment