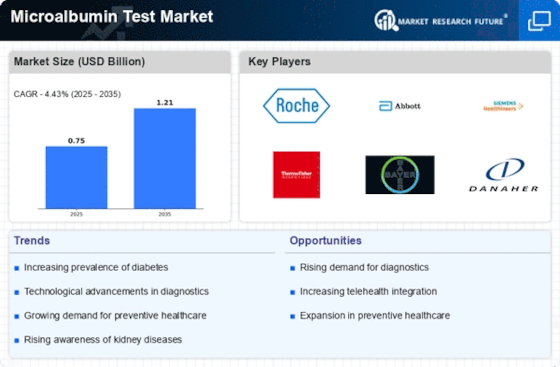
Top Industry Leaders in the Microalbumin Test Market

Latest Microalbumin Test Companies Updates:
Roche Diagnostics Launched their cobas u 702 analyzer, a compact and automated system for various lab tests, including microalbumin-to-creatinine ratio (UACR) testing.Collaborated with research institutions on developing next-generation microalbumin assays with improved sensitivity and specificity for early CKD detection.
Abbott Laboratories Received FDA approval for their Alinity i-QMRA UACR test, offering on-demand measurement of UACR on their Alinity i-QMRA instrument in clinical settings.Partnering with healthcare providers to implement point-of-care UACR testing devices for rapid diagnosis and early CKD management.
Siemens Healthcare Introduced their Atellica ADVIA Centaur XP immunoassay system, featuring a microalbumin assay with improved analytical performance and faster turnaround times.Investing in developing automated platforms for integrated analysis of various kidney function markers, including microalbumin.
Randox Laboratories Developed a portable UACR testing device for point-of-care use in resource-limited settings, facilitating access to early CKD diagnosis in underserved communities.Partnering with non-profit organizations to promote awareness and screening programs for CKD and diabetes, leading to increased utilization of microalbumin testing.
List of Microalbumin Test Companies Key companies in the market
- Beckman Coulter
- Abbott
- Nova Biomedical
- Siemens
- ARKRAY
- ACON Laboratories
- Sysmex