Rising Prevalence of Chronic Diseases
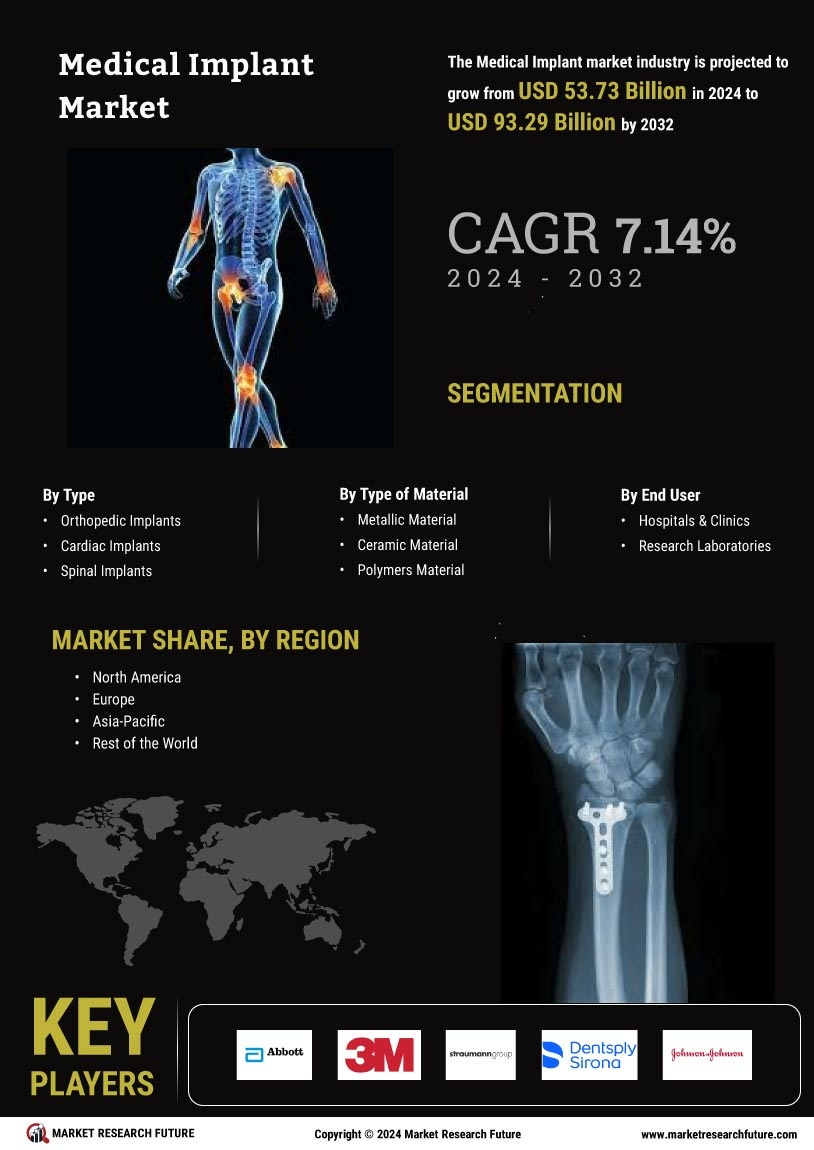
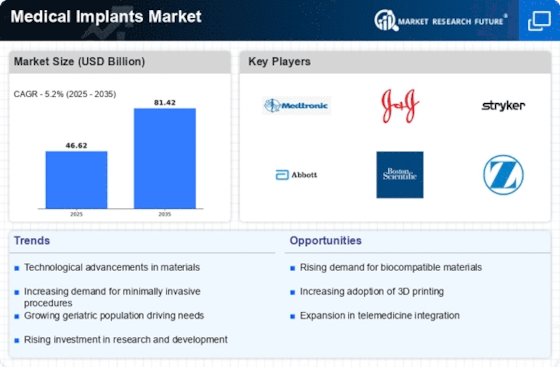
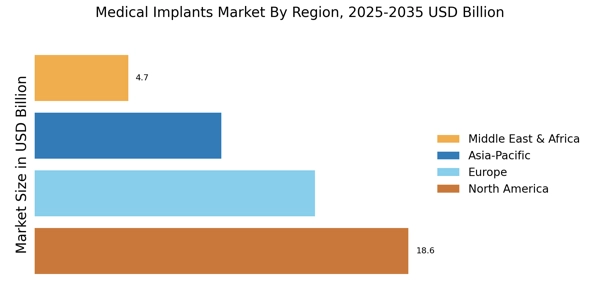
The increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders, and orthopedic conditions is a primary driver of the Medical Implants Market. As these conditions become more common, the demand for medical implants, including stents, pacemakers, and joint replacements, is likely to rise. According to recent data, the number of individuals requiring orthopedic implants is projected to reach 3 million annually by 2025. This trend indicates a growing need for innovative solutions in the Medical Implants Market, as healthcare providers seek to improve patient outcomes and enhance quality of life. Furthermore, the aging population is expected to contribute significantly to this demand, as older adults are more susceptible to chronic ailments that necessitate surgical interventions involving implants.
Technological Advancements in Implant Design
Technological advancements in implant design and materials are transforming the Medical Implants Market. Innovations such as 3D printing, biocompatible materials, and smart implants are enhancing the functionality and effectiveness of medical devices. For instance, the introduction of bioresorbable stents has revolutionized cardiovascular treatments, allowing for improved patient recovery and reduced complications. The market for orthopedic implants is also experiencing growth, with projections indicating a compound annual growth rate of 6.5% through 2025. These advancements not only improve surgical outcomes but also reduce the overall cost of healthcare, making them attractive to both providers and patients. As technology continues to evolve, the Medical Implants Market is likely to witness further enhancements that could lead to more personalized and effective treatment options.
Increasing Investment in Healthcare Infrastructure
The increasing investment in healthcare infrastructure is a significant driver of the Medical Implants Market. Governments and private entities are allocating substantial resources to enhance healthcare facilities, which includes the procurement of advanced medical technologies and implants. For example, investments in surgical centers and hospitals are expected to rise, leading to an increased demand for various medical implants. In 2025, the healthcare expenditure is projected to reach approximately 10 trillion dollars, with a notable portion directed towards improving surgical capabilities. This trend suggests that as healthcare systems expand and modernize, the Medical Implants Market will benefit from heightened demand for innovative implant solutions that cater to a broader patient base.
Aging Population and Demand for Surgical Interventions
The aging population is a critical driver of the Medical Implants Market, as older adults often require surgical interventions that involve implants. With a significant portion of the population reaching retirement age, the demand for orthopedic, dental, and cardiovascular implants is likely to surge. Projections indicate that by 2025, nearly 1.5 billion individuals will be aged 65 and older, many of whom will require medical implants to address age-related health issues. This demographic shift suggests a robust market potential for implant manufacturers, as healthcare systems adapt to meet the needs of an aging population. Consequently, the Medical Implants Market is poised for growth, driven by the necessity for effective solutions that enhance the quality of life for older adults.
Growing Awareness and Acceptance of Implant Procedures
Growing awareness and acceptance of implant procedures among patients and healthcare professionals is driving the Medical Implants Market. Educational initiatives and marketing efforts by medical device companies are enhancing understanding of the benefits and risks associated with implants. As patients become more informed, they are more likely to consider surgical options involving implants for conditions such as joint pain or heart disease. Surveys indicate that over 70% of patients express a willingness to undergo implant procedures when adequately informed about their advantages. This shift in perception is likely to lead to increased adoption rates of various implants, thereby propelling the growth of the Medical Implants Market. Furthermore, as healthcare providers advocate for these procedures, the market is expected to expand significantly.