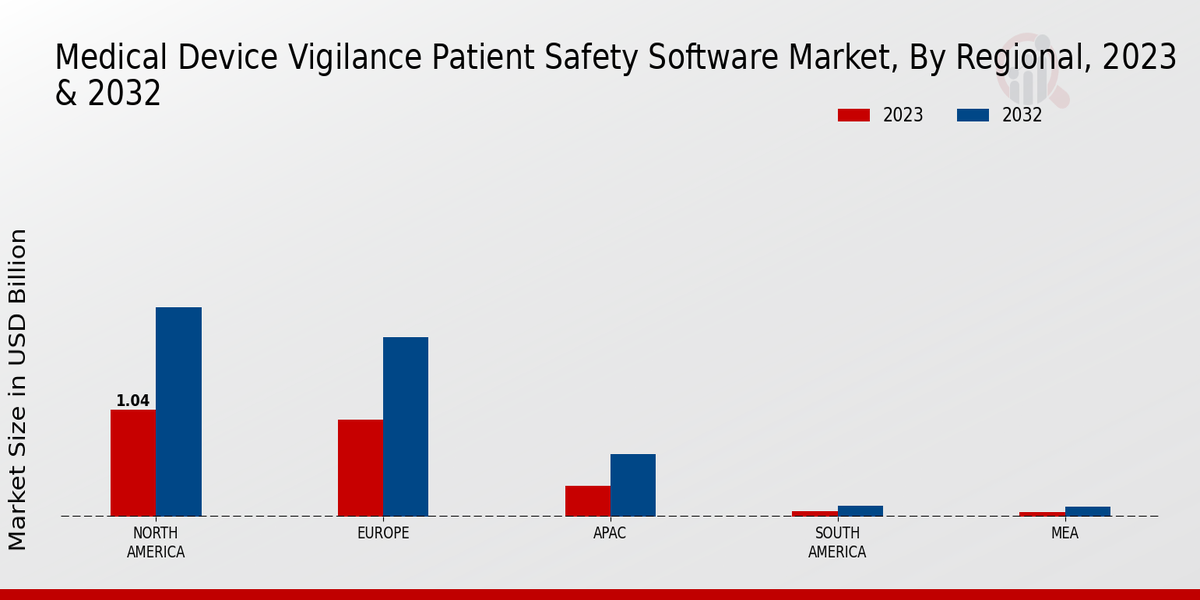
Market Growth Projections
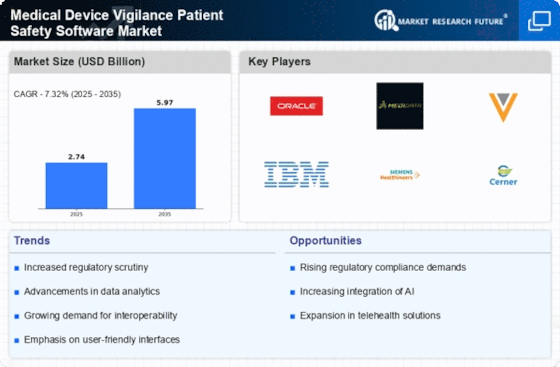
The Global Medical Device Vigilance Patient Safety Software Market Industry is poised for substantial growth, with projections indicating a rise from 2.74 USD Billion in 2024 to 5.97 USD Billion by 2035. This growth trajectory reflects the increasing recognition of the importance of patient safety and the need for effective vigilance systems. The anticipated compound annual growth rate (CAGR) of 7.33% from 2025 to 2035 underscores the market's potential as stakeholders invest in advanced software solutions to enhance safety protocols. This upward trend is likely to attract new entrants and drive innovation within the industry, further solidifying the role of vigilance software in ensuring patient safety.
Technological Advancements
Technological innovations are transforming the landscape of the Global Medical Device Vigilance Patient Safety Software Market Industry. The integration of artificial intelligence and machine learning into vigilance software is enabling more efficient data processing and analysis. These advancements facilitate the identification of potential safety issues before they escalate, thereby improving patient outcomes. As healthcare systems increasingly adopt these technologies, the market is likely to witness significant growth. The anticipated compound annual growth rate (CAGR) of 7.33% from 2025 to 2035 reflects the potential of these innovations to reshape the industry and enhance the effectiveness of patient safety measures.
Growing Focus on Patient Safety
The emphasis on patient safety is a pivotal driver in the Global Medical Device Vigilance Patient Safety Software Market Industry. Healthcare providers are increasingly prioritizing the implementation of systems that can effectively monitor adverse events and ensure timely reporting. This focus is underscored by initiatives from health organizations advocating for improved patient safety protocols. Consequently, the demand for vigilance software that can provide real-time data analytics and risk assessment is on the rise. This trend is anticipated to propel the market forward, as stakeholders recognize the value of investing in technologies that enhance patient safety outcomes.
Increasing Regulatory Requirements
The Global Medical Device Vigilance Patient Safety Software Market Industry is experiencing heightened regulatory scrutiny, compelling manufacturers to adopt robust vigilance systems. Regulatory bodies worldwide, including the FDA and EMA, are enforcing stricter compliance measures to ensure patient safety. This trend is likely to drive the demand for sophisticated software solutions that can streamline reporting and monitoring processes. As a result, companies are investing in advanced vigilance software to meet these evolving standards, thereby enhancing their market position. This shift is expected to contribute to the market's growth, with projections indicating a rise from 2.74 USD Billion in 2024 to 5.97 USD Billion by 2035.
Rising Incidence of Adverse Events
The rising incidence of adverse events associated with medical devices is a critical factor driving the Global Medical Device Vigilance Patient Safety Software Market Industry. As the complexity of medical devices increases, so does the potential for complications and safety issues. This trend has prompted healthcare organizations to seek comprehensive vigilance solutions that can effectively monitor and report adverse events. The increasing awareness of these incidents among patients and healthcare professionals is likely to further fuel demand for vigilance software. Consequently, this heightened focus on monitoring adverse events is expected to contribute to the market's growth trajectory in the coming years.
Expansion of the Medical Device Market
The expansion of the global medical device market is significantly influencing the Global Medical Device Vigilance Patient Safety Software Market Industry. As new devices are developed and introduced, the need for effective vigilance systems to monitor their safety and efficacy becomes paramount. This growth is driven by advancements in technology and an aging population requiring more medical interventions. The increasing number of devices on the market necessitates robust software solutions to ensure compliance with safety regulations and to protect patient welfare. This trend is projected to support the market's growth, with an expected increase from 2.74 USD Billion in 2024 to 5.97 USD Billion by 2035.



















Leave a Comment