Regulatory Compliance and Quality Standards
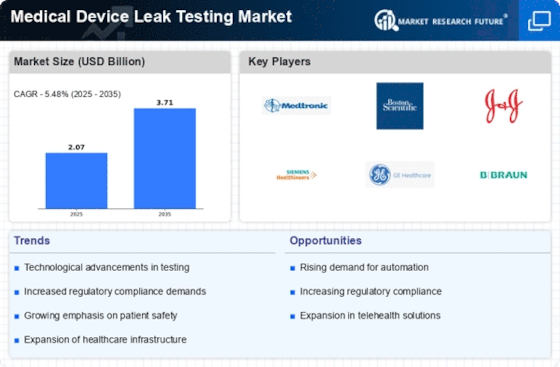
Regulatory compliance remains a pivotal driver in the Medical Device Leak Testing Market. Stringent regulations imposed by health authorities necessitate rigorous testing of medical devices to ensure safety and efficacy. Compliance with standards such as ISO 13485 and FDA regulations compels manufacturers to adopt advanced leak testing methodologies. The increasing emphasis on quality assurance is reflected in the market, where the demand for reliable testing solutions is on the rise. As manufacturers strive to adhere to these regulations, investments in leak testing technologies are expected to escalate, further propelling market growth. This trend underscores the critical role of leak testing in maintaining product quality and patient safety.
Growing Aging Population and Chronic Diseases
The Medical Device Leak Testing Market is significantly influenced by the growing aging population and the prevalence of chronic diseases. As the demographic landscape shifts, there is an increasing need for medical devices that cater to the health requirements of older adults. This demographic shift is likely to drive the demand for reliable medical devices, necessitating rigorous leak testing to ensure their safety and effectiveness. The market is expected to grow as manufacturers respond to this demand by investing in advanced leak testing technologies. This trend highlights the importance of ensuring device integrity in the face of rising healthcare challenges associated with aging.
Rising Demand for Minimally Invasive Procedures
The Medical Device Leak Testing Market is witnessing a notable increase in demand for minimally invasive procedures. As healthcare providers and patients alike favor less invasive options, the need for reliable medical devices that ensure patient safety becomes paramount. Leak testing is essential in validating the integrity of devices used in these procedures, such as catheters and stents. The market is projected to expand as manufacturers focus on developing innovative devices that meet the growing demand for minimally invasive solutions. This shift not only enhances patient outcomes but also drives the need for robust leak testing protocols to ensure device reliability.
Technological Advancements in Testing Equipment
The Medical Device Leak Testing Market is experiencing a surge in technological advancements that enhance testing accuracy and efficiency. Innovations such as automated leak testing systems and advanced sensors are being integrated into testing protocols. These technologies not only reduce human error but also increase throughput, allowing manufacturers to meet stringent production demands. The market for leak testing equipment is projected to grow significantly, with estimates suggesting a compound annual growth rate of over 6% in the coming years. This growth is driven by the need for precise testing methods that ensure the integrity of medical devices, thereby fostering trust among healthcare providers and patients alike.
Increased Investment in Healthcare Infrastructure
Investment in healthcare infrastructure is a crucial driver for the Medical Device Leak Testing Market. Governments and private entities are allocating substantial resources to enhance healthcare facilities, which includes upgrading medical device testing capabilities. This investment is likely to lead to the adoption of advanced leak testing technologies, ensuring that medical devices meet the highest safety standards. As healthcare systems expand and modernize, the demand for reliable testing solutions is expected to rise. This trend not only supports the growth of the leak testing market but also contributes to improved patient outcomes through enhanced device reliability.