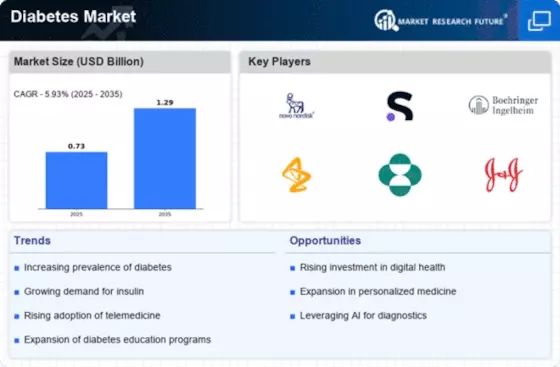
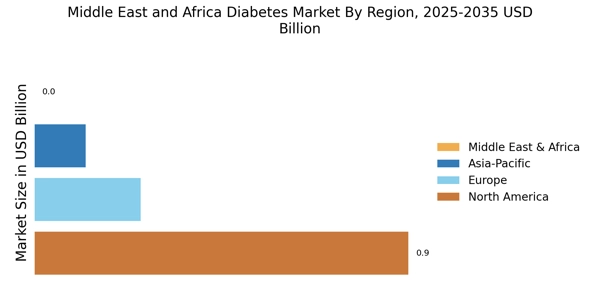
Rising Healthcare Expenditure
Healthcare expenditure in the MEA region is on an upward trajectory, which is likely to bolster the MEA Diabetes Market. Governments are increasingly allocating funds to improve healthcare infrastructure and access to diabetes care. For example, countries like Saudi Arabia and the UAE are investing heavily in healthcare facilities and diabetes management programs. According to recent data, healthcare spending in the Middle East is expected to reach USD 104 billion by 2025. This increase in expenditure is anticipated to enhance the availability of diabetes medications, devices, and educational resources, thereby supporting the growth of the MEA Diabetes Market.
Enhanced Regulatory Frameworks
The MEA Diabetes Market is benefiting from enhanced regulatory frameworks aimed at improving diabetes care and management. Governments in the region are implementing stricter regulations on the quality and safety of diabetes medications and devices. For instance, the Gulf Cooperation Council (GCC) has established guidelines to ensure that diabetes products meet international standards. These regulatory improvements are likely to foster consumer confidence and encourage the adoption of diabetes management solutions. As a result, the MEA Diabetes Market is expected to experience growth as healthcare providers and patients increasingly rely on regulated and effective diabetes care options.
Increasing Awareness and Education
The MEA Diabetes Market is experiencing a notable increase in awareness and education regarding diabetes management. Public health campaigns and educational programs are being implemented across various countries in the Middle East and Africa. These initiatives aim to inform the population about diabetes risk factors, symptoms, and the importance of early diagnosis. For instance, the prevalence of diabetes in the region is projected to rise significantly, with estimates suggesting that by 2045, approximately 55 million adults in the MEA region will be living with diabetes. This growing awareness is likely to drive demand for diabetes care products and services, thereby positively impacting the MEA Diabetes Market.
Aging Population and Lifestyle Changes
The demographic shift towards an aging population in the MEA region is contributing to the growth of the MEA Diabetes Market. Older adults are at a higher risk of developing diabetes due to age-related factors. Additionally, lifestyle changes, including urbanization and dietary shifts, are leading to increased obesity rates, which is a significant risk factor for diabetes. Reports indicate that the number of people aged 60 and above in the MEA region is expected to double by 2050. This demographic trend is likely to result in a higher prevalence of diabetes, thereby driving demand for diabetes care products and services within the MEA Diabetes Market.
Growing Demand for Innovative Diabetes Management Solutions
The MEA Diabetes Market is witnessing a surge in demand for innovative diabetes management solutions. Technological advancements, such as continuous glucose monitoring systems and insulin delivery devices, are becoming increasingly popular among patients. The market for diabetes devices in the MEA region is projected to grow at a compound annual growth rate (CAGR) of 8.5% from 2021 to 2026. This trend indicates a shift towards more effective and user-friendly diabetes management options, which could significantly enhance patient outcomes and adherence to treatment plans. As a result, the MEA Diabetes Market is likely to benefit from the introduction of these advanced solutions.