Expansion of Point-of-Care Testing
The expansion of point-of-care testing (POCT) is a significant driver for the Lateral Flow Assay Market. With the increasing need for rapid diagnostics in various settings, including clinics, pharmacies, and even at home, lateral flow assays are gaining traction. The convenience of obtaining immediate results without the need for laboratory infrastructure is appealing to both healthcare providers and patients. Market analysis suggests that the POCT segment is expected to witness substantial growth, with lateral flow assays being at the forefront of this trend. This shift towards decentralized testing solutions is likely to enhance the overall market landscape, making diagnostics more accessible to diverse populations.
Growing Focus on Preventive Healthcare
The increasing emphasis on preventive healthcare is driving the Lateral Flow Assay Market. As populations become more health-conscious, there is a growing preference for early detection of diseases through regular screening. Lateral flow assays, known for their ease of use and rapid results, are becoming integral to preventive health strategies. According to recent studies, the market for preventive diagnostics is expected to expand significantly, with lateral flow assays being a preferred choice due to their cost-effectiveness and accessibility. This trend indicates a shift in healthcare paradigms, where proactive measures are prioritized, thereby enhancing the demand for lateral flow testing solutions.
Increasing Prevalence of Infectious Diseases
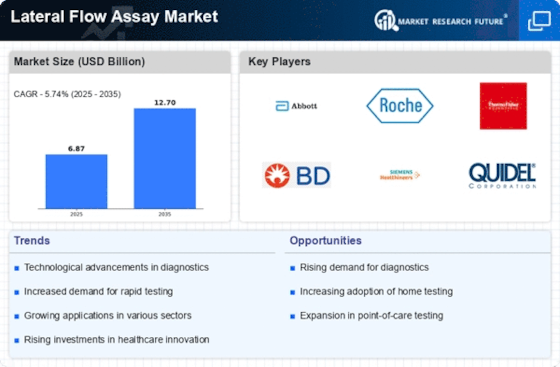
The rising incidence of infectious diseases is a primary driver for the Lateral Flow Assay Market. As healthcare systems strive to manage outbreaks effectively, the demand for rapid diagnostic tools has surged. For instance, the World Health Organization has reported a notable increase in diseases such as influenza and hepatitis, necessitating swift testing solutions. This trend is expected to propel the Lateral Flow Assay Market, as these assays provide quick results, enabling timely treatment decisions. Furthermore, the market is projected to grow at a compound annual growth rate of approximately 8% over the next few years, reflecting the urgent need for efficient diagnostic methods in various healthcare settings.
Rising Investment in Healthcare Infrastructure
The surge in investment in healthcare infrastructure is positively influencing the Lateral Flow Assay Market. Governments and private entities are increasingly allocating funds to enhance healthcare facilities, particularly in developing regions. This investment is aimed at improving diagnostic capabilities, which includes the adoption of lateral flow assays for their rapid and reliable results. As healthcare systems evolve, the demand for efficient diagnostic tools is expected to rise, thereby driving market growth. Reports indicate that the healthcare sector is projected to receive significant funding over the next few years, which could lead to an increased adoption of lateral flow assays in various healthcare settings.
Technological Innovations in Assay Development
Technological advancements play a crucial role in shaping the Lateral Flow Assay Market. Innovations such as enhanced sensitivity and specificity in assay design have significantly improved diagnostic accuracy. The integration of microfluidics and nanotechnology has led to the development of more sophisticated assays that can detect multiple pathogens simultaneously. This evolution not only broadens the application scope of lateral flow assays but also attracts investments from key players in the healthcare sector. As a result, the market is witnessing a shift towards more advanced and user-friendly testing solutions, which could potentially increase market penetration and adoption rates across various demographics.