Market Growth Projections
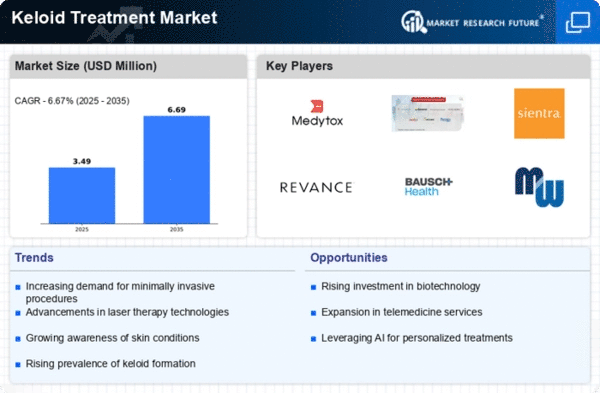
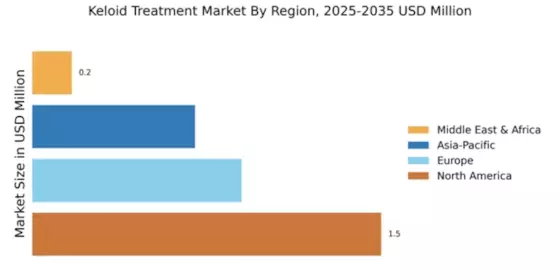
The Global Keloid Treatment Market Industry is projected to experience steady growth, with estimates indicating a market size of 1.73 USD Billion in 2024 and a potential increase to 1.9 USD Billion by 2035. This growth trajectory suggests a compound annual growth rate (CAGR) of 0.86% from 2025 to 2035. Such projections highlight the ongoing demand for keloid treatments and the industry's responsiveness to emerging trends and technologies. The market's expansion reflects broader healthcare trends, including a focus on personalized medicine and improved patient care.
Rising Cosmetic Procedures
The rise in cosmetic procedures is another driver for the Global Keloid Treatment Market Industry. As more individuals undergo surgeries for aesthetic enhancements, the likelihood of keloid formation increases. This trend is particularly pronounced in regions where cosmetic surgery is gaining popularity. Patients seeking to minimize the appearance of keloids post-surgery are driving demand for effective treatment solutions. The market's growth is expected to be sustained as the beauty industry continues to expand, with keloid treatments becoming an integral part of post-operative care.
Rising Prevalence of Keloids
The Global Keloid Treatment Market Industry is experiencing growth due to the rising prevalence of keloids, which are often associated with surgical scars, acne, and trauma. As awareness increases regarding the condition, more individuals seek treatment options. In 2024, the market is projected to reach 1.73 USD Billion, reflecting a growing demand for effective therapies. This trend is particularly evident in regions with high rates of skin injuries, such as urban areas where surgical procedures are common. The increasing incidence of keloids is likely to drive innovation in treatment modalities, further expanding the market.
Growing Awareness and Education
The Global Keloid Treatment Market Industry benefits from increasing awareness and education about keloids among both healthcare professionals and patients. Educational campaigns and resources provided by dermatological associations are helping to demystify keloids, leading to earlier diagnosis and treatment. This heightened awareness is crucial, as it encourages individuals to seek medical advice sooner, thereby increasing treatment uptake. As more patients become informed about their options, the market is likely to see a steady rise in demand for keloid treatments, contributing to a projected CAGR of 0.86% from 2025 to 2035.
Increased Research and Development
Increased investment in research and development is propelling the Global Keloid Treatment Market Industry forward. Pharmaceutical companies and research institutions are focusing on understanding the underlying mechanisms of keloid formation, which may lead to the discovery of novel therapeutic agents. This emphasis on R&D is likely to yield new treatment options that are more effective and tailored to individual patient needs. As the market evolves, the introduction of innovative therapies could significantly alter the treatment landscape, enhancing patient satisfaction and outcomes.
Advancements in Treatment Technologies
Innovations in treatment technologies are significantly influencing the Global Keloid Treatment Market Industry. New methods, such as laser therapy, cryotherapy, and intralesional injections, are being developed and refined, offering patients more effective options. These advancements not only enhance treatment efficacy but also reduce recovery times and side effects. As a result, healthcare providers are increasingly adopting these technologies, contributing to market growth. The anticipated increase in market size to 1.9 USD Billion by 2035 underscores the potential of these advancements to reshape treatment paradigms and improve patient outcomes.