Focus on Cost Efficiency
Cost efficiency remains a critical driver for the life sciences-bpo market in Japan. Companies are increasingly pressured to minimize operational costs while maintaining high-quality standards. Outsourcing non-core functions allows organizations to achieve significant savings, with estimates suggesting that firms can reduce costs by up to 30% through strategic partnerships with BPO providers. This focus on cost efficiency is particularly relevant in the context of rising R&D expenditures in the pharmaceutical sector. As companies strive to bring new drugs to market faster, the life sciences-bpo market is positioned to thrive by offering tailored solutions that align with budgetary constraints. This trend underscores the importance of financial prudence in the decision-making processes of life sciences firms.
Regulatory Landscape Adaptation
The regulatory landscape in Japan is complex and continuously evolving, which significantly impacts the life sciences-bpo market. Companies must navigate stringent regulations related to drug approval, clinical trials, and data privacy. BPO providers are increasingly seen as essential partners in ensuring compliance with these regulations. By leveraging their expertise, organizations can mitigate risks associated with non-compliance, which can lead to costly penalties and delays. In 2025, it is anticipated that the demand for regulatory affairs outsourcing will grow by approximately 15%, as firms seek to adapt to changing regulations efficiently. This adaptation not only enhances operational resilience but also positions the life sciences-bpo market as a critical component in the broader life sciences ecosystem.
Emphasis on Quality and Compliance
Quality assurance and compliance are paramount in the life sciences-bpo market in Japan. As companies face increasing scrutiny from regulatory bodies, the demand for high-quality services is intensifying. BPO providers are expected to implement robust quality management systems to meet these expectations. In 2025, it is projected that around 70% of life sciences firms will prioritize partnerships with BPO providers that demonstrate a commitment to quality and compliance. This emphasis on quality not only ensures patient safety but also enhances the credibility of the organizations involved. Consequently, the life sciences-bpo market is likely to evolve, with providers focusing on delivering superior services that align with industry standards and regulatory requirements.
Rising Demand for Outsourced Services
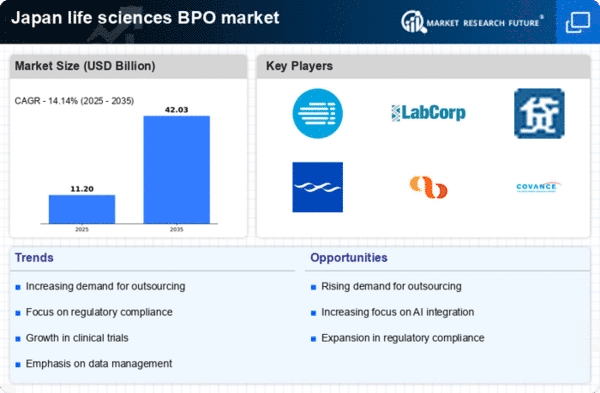
The life sciences-bpo market in Japan experiences a notable increase in demand for outsourced services. This trend is driven by pharmaceutical and biotechnology companies seeking to enhance operational efficiency and reduce costs. As organizations focus on core competencies, they are increasingly turning to BPO providers for non-core functions such as clinical trials, data management, and regulatory affairs. In 2025, the market is projected to grow at a CAGR of approximately 8.5%, reflecting the growing reliance on external expertise. This shift allows companies to allocate resources more effectively while ensuring compliance with stringent regulations. The life sciences-bpo market is thus positioned to benefit from this rising demand, as firms look to leverage specialized knowledge and technology to streamline processes.
Technological Integration and Innovation
Technological integration plays a pivotal role in shaping the life sciences-bpo market in Japan. The adoption of advanced technologies such as artificial intelligence, machine learning, and big data analytics is transforming how BPO services are delivered. These innovations enable more efficient data processing, improved patient engagement, and enhanced decision-making capabilities. In 2025, it is estimated that around 60% of BPO providers will incorporate AI-driven solutions to optimize their service offerings. This technological evolution not only enhances operational efficiency but also supports compliance with regulatory requirements. As a result, the life sciences-bpo market is likely to see increased investment in technology, fostering a competitive landscape that prioritizes innovation and quality.