Advancements in Genomic Research
The rapid advancements in genomic research are transforming the landscape of the biomarker test market. Japan has made significant strides in genomics, with numerous research institutions focusing on the identification of novel biomarkers linked to various diseases. This research is expected to yield innovative testing solutions that enhance diagnostic precision and treatment efficacy. The biomarker test market is poised for growth as these advancements translate into clinically relevant tests that can be integrated into routine healthcare practices. The collaboration between academia and industry is likely to accelerate the development of new biomarker tests, further driving market expansion.
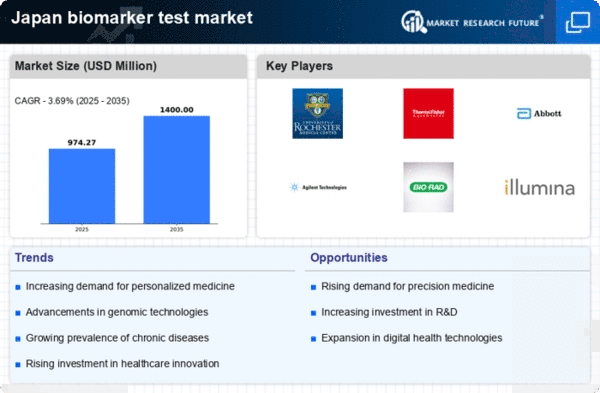
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases in Japan is a pivotal driver for the biomarker test market. As the population ages, conditions such as cancer, diabetes, and cardiovascular diseases become more prevalent. This trend necessitates advanced diagnostic tools, including biomarker tests, to facilitate early detection and personalized treatment strategies. According to recent statistics, chronic diseases account for approximately 60% of all deaths in Japan, underscoring the urgent need for effective diagnostic solutions. The biomarker test market is thus positioned to expand significantly as healthcare providers seek to implement these tests to improve patient outcomes and reduce healthcare costs.
Rising Awareness of Preventive Healthcare
There is a notable shift towards preventive healthcare in Japan, which significantly influences the biomarker test market. As individuals become more health-conscious, there is an increasing demand for tests that can identify potential health risks before they develop into serious conditions. This trend is reflected in the growing number of health screenings and wellness programs offered by employers and healthcare providers. The biomarker test market is likely to thrive as more people seek proactive measures to monitor their health, leading to a higher uptake of biomarker tests for early detection and risk assessment.
Regulatory Support for Innovative Diagnostics
The regulatory environment in Japan is becoming increasingly supportive of innovative diagnostic solutions, which is beneficial for the biomarker test market. The Pharmaceuticals and Medical Devices Agency (PMDA) has streamlined approval processes for new diagnostic tests, encouraging the introduction of cutting-edge biomarker tests. This regulatory support is crucial for fostering innovation and ensuring that patients have access to the latest diagnostic technologies. As a result, the biomarker test market is expected to experience accelerated growth, as companies are incentivized to develop and commercialize novel tests that meet the evolving needs of the healthcare system.
Growing Investment in Healthcare Infrastructure
Japan's commitment to enhancing its healthcare infrastructure plays a crucial role in the growth of the biomarker test market. The government has allocated substantial funding to modernize healthcare facilities and integrate advanced diagnostic technologies. In 2025, healthcare expenditure is projected to reach ¥42 trillion, with a significant portion directed towards innovative diagnostic solutions. This investment is likely to foster the adoption of biomarker tests, as healthcare providers increasingly recognize their value in improving diagnostic accuracy and patient management. The biomarker test market stands to benefit from this trend, as enhanced infrastructure facilitates the implementation of cutting-edge testing methodologies.