Regulatory Framework Enhancements
The regulatory landscape in Italy is evolving to support the growth of the chemiluminescence immunoassay-analyzers market. Recent updates to regulations governing medical devices and diagnostic tests are likely to streamline the approval process for new technologies. This regulatory support is expected to encourage manufacturers to invest in research and development, leading to the introduction of innovative products in the market. The Italian Medicines Agency (AIFA) has been actively working to ensure that regulations keep pace with technological advancements, which may facilitate quicker market entry for new analyzers. As a result, the chemiluminescence immunoassay-analyzers market could see an influx of advanced products, enhancing competition and driving growth.
Rising Demand for Diagnostic Testing
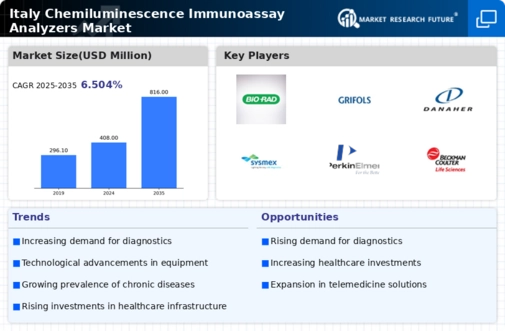
The increasing prevalence of chronic diseases in Italy is driving the demand for diagnostic testing, which in turn fuels the growth of the chemiluminescence immunoassay-analyzers market. As healthcare providers seek efficient and accurate testing methods, the adoption of chemiluminescence immunoassay technology is likely to rise. Reports indicate that the market for diagnostic testing in Italy is projected to reach €2 billion by 2026, highlighting the potential for chemiluminescence immunoassay-analyzers to capture a significant share of this expanding market. Furthermore, the emphasis on early disease detection and management is expected to enhance the utilization of these analyzers, thereby contributing to the overall growth of the industry.
Growing Awareness of Preventive Healthcare
There is a notable shift towards preventive healthcare in Italy, which is influencing the chemiluminescence immunoassay-analyzers market. As the population becomes more health-conscious, there is an increasing demand for routine health screenings and early detection of diseases. This trend is likely to drive the adoption of chemiluminescence immunoassay technology, as it offers rapid and reliable results. According to recent surveys, approximately 70% of Italians express a preference for preventive health measures, suggesting a robust market potential for analyzers that facilitate such initiatives. Consequently, the chemiluminescence immunoassay-analyzers market is expected to benefit from this growing awareness and demand for preventive healthcare solutions.
Technological Innovations in Assay Development
The chemiluminescence immunoassay-analyzers market is experiencing a wave of technological innovations that are enhancing assay development and performance. Advances in reagent formulations and detection technologies are likely to improve the sensitivity and specificity of tests, making them more appealing to healthcare providers. In Italy, research institutions and companies are collaborating to develop next-generation analyzers that can deliver faster results with minimal sample volumes. This innovation is expected to attract more laboratories to adopt chemiluminescence immunoassay technology, thereby expanding the market. The continuous evolution of assay methodologies suggests a promising future for the industry as it adapts to the changing needs of healthcare.
Increased Investment in Healthcare Infrastructure
Italy's government has been making substantial investments in healthcare infrastructure, which is anticipated to positively impact the chemiluminescence immunoassay-analyzers market. Enhanced laboratory facilities and the modernization of existing healthcare systems are likely to facilitate the adoption of advanced diagnostic technologies. The Italian Ministry of Health has allocated approximately €1.5 billion for healthcare upgrades in the current fiscal year, indicating a strong commitment to improving healthcare services. This investment is expected to create a conducive environment for the deployment of chemiluminescence immunoassay-analyzers, as laboratories seek to enhance their testing capabilities and improve patient outcomes.






















Leave a Comment