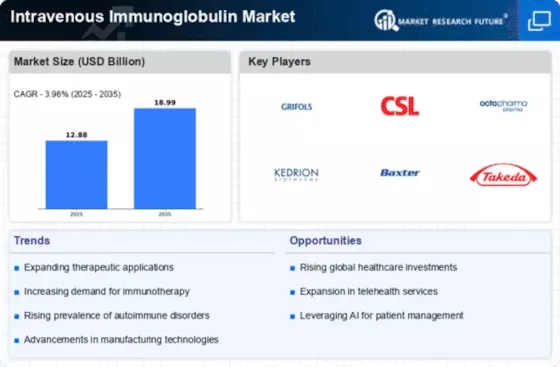
Top Industry Leaders in the Intravenous Immunoglobulin Market

Biotest AG receives EU approval for Yimmugo (IgG Next Generation) sugar-free, ready-to-use solution offers a new IVIG option for primary antibody deficiency syndromes, secondary immune deficiency, and immunomodulation in autoimmune diseases.
Takeda launches Hizentra Plus new formulation combines Hizentra (immune globulin intravenous, human) with hyaluronidase for subcutaneous administration, providing an alternative delivery option for patients with needle phobia or venous access challenges.
CSL Behring expands its portfolio with Cuvitru FDA-approved subcutaneous IVIG option offers another potential avenue for convenient and flexible administration, improving patient compliance and quality of life.
Companies like Octapharma and Grifols are developing and expanding their pediatric IVIG offerings, addressing the specific requirements and safety considerations for younger patients.
List of Intravenous Immunoglobulin Key Companies in the Market
-
Kedrion S.p.A
-
Baxter International Inc
-
OMRIX Biopharmaceuticals Ltd
-
Biotest AG
-
Hualan Biological Engineering Inc
-
Shanghai RAAS Blood Products Co
-
CSL Limited
-
Grifols
-
Guizhou Taibang Biological Products Co