Market Growth Projections
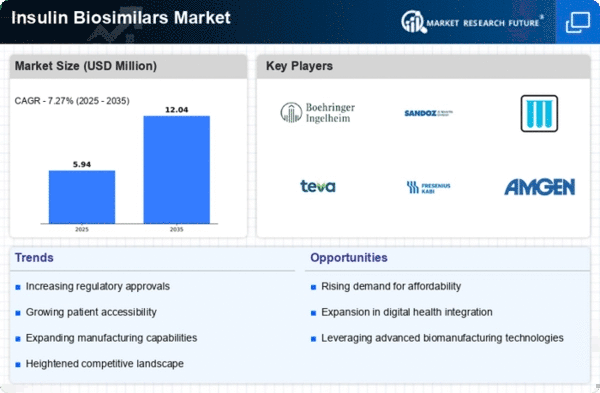
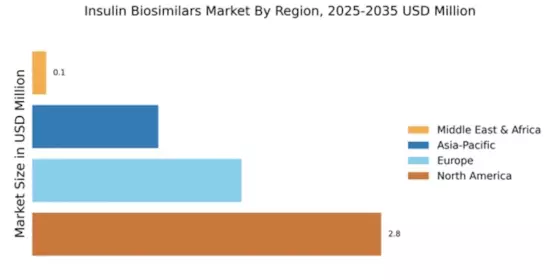
The Global Insulin Biosimilars Market Industry is projected to experience substantial growth in the coming years. With a market size of 0.52 USD Billion in 2024, it is expected to expand significantly, reaching 2.53 USD Billion by 2035. This growth trajectory indicates a compound annual growth rate of 15.43% from 2025 to 2035. Such projections reflect the increasing demand for insulin therapies, driven by factors such as rising diabetes prevalence and the growing acceptance of biosimilars. The market dynamics suggest a robust future for biosimilars, positioning them as a key component in diabetes management.
Rising Prevalence of Diabetes
The increasing incidence of diabetes globally is a primary driver for the Global Insulin Biosimilars Market Industry. As of 2024, the number of individuals diagnosed with diabetes is projected to reach approximately 537 million, a figure that underscores the urgent need for effective insulin therapies. This growing patient population is expected to drive demand for biosimilars, which offer a cost-effective alternative to traditional insulin products. The market is anticipated to expand significantly, with projections indicating a rise to 2.53 USD Billion by 2035, reflecting a compound annual growth rate of 15.43% from 2025 to 2035.
Cost-Effectiveness of Biosimilars
The cost-effectiveness of insulin biosimilars plays a crucial role in shaping the Global Insulin Biosimilars Market Industry. With healthcare costs rising, biosimilars present a more affordable option for patients and healthcare systems alike. They typically offer savings of 20-30% compared to their reference products, which can alleviate the financial burden on patients and insurers. As healthcare providers increasingly prioritize cost management, the adoption of biosimilars is likely to accelerate. This trend is particularly relevant in regions with high diabetes prevalence, where the demand for affordable insulin therapies is paramount.
Regulatory Support and Frameworks
Regulatory bodies worldwide are establishing supportive frameworks for the approval and commercialization of insulin biosimilars, thereby bolstering the Global Insulin Biosimilars Market Industry. Initiatives aimed at streamlining the approval process and ensuring the safety and efficacy of biosimilars have been implemented in various countries. For instance, the European Medicines Agency has developed guidelines that facilitate the entry of biosimilars into the market. This regulatory support not only enhances market confidence but also encourages investment in biosimilar development, potentially leading to a broader range of products available to patients.
Technological Advancements in Biomanufacturing
Technological advancements in biomanufacturing are significantly influencing the Global Insulin Biosimilars Market Industry. Innovations in production processes, such as improved cell line development and purification techniques, have enhanced the efficiency and yield of biosimilar insulin products. These advancements not only reduce production costs but also improve the quality and consistency of the final products. As manufacturers leverage these technologies, the availability of high-quality biosimilars is expected to increase, further driving market growth. The anticipated market size of 0.52 USD Billion in 2024 reflects the early stages of this technological evolution.
Growing Awareness and Acceptance of Biosimilars
The growing awareness and acceptance of biosimilars among healthcare professionals and patients are pivotal for the Global Insulin Biosimilars Market Industry. Educational initiatives and outreach programs have been instrumental in dispelling misconceptions about biosimilars, leading to increased confidence in their use. As healthcare providers become more familiar with the benefits and safety profiles of biosimilars, they are more likely to prescribe these alternatives to traditional insulin therapies. This shift in perception is crucial for market expansion, as it fosters a more favorable environment for biosimilar adoption.