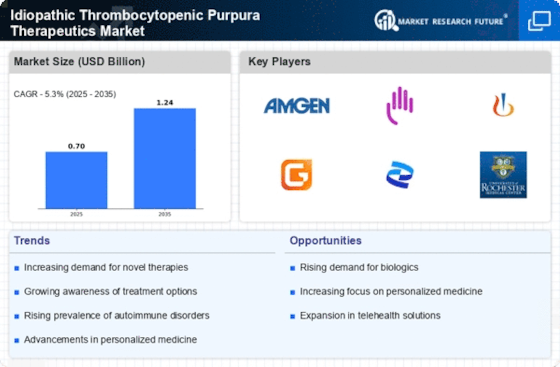
The Idiopathic Thrombocytopenic Purpura (ITP) Therapeutics Market is characterized by a dynamic competitive landscape, driven by a combination of innovative therapies, strategic partnerships, and a growing emphasis on patient-centric solutions. Key players such as Amgen (US), Bristol-Myers Squibb (US), and Novartis (CH) are actively shaping the market through their distinct operational focuses. Amgen (US) has been concentrating on the development of biologics, particularly in the realm of monoclonal antibodies, which are increasingly recognized for their efficacy in treating ITP. Meanwhile, Bristol-Myers Squibb (US) is leveraging its expertise in immunotherapy to explore novel treatment pathways, while Novartis (CH) is focusing on expanding its portfolio through strategic acquisitions and collaborations, thereby enhancing its market presence. Collectively, these strategies contribute to a competitive environment that is both concentrated and evolving, as companies seek to differentiate themselves through innovation and strategic positioning.
In terms of business tactics, companies are increasingly localizing manufacturing and optimizing supply chains to enhance efficiency and responsiveness to market demands. The ITP therapeutics market appears moderately fragmented, with several players vying for market share. However, the influence of major companies is substantial, as they not only drive innovation but also set industry standards that smaller firms often follow. This competitive structure suggests that while there is room for new entrants, established players maintain a significant advantage due to their resources and market knowledge.
In August 2025, Amgen (US) announced a strategic partnership with a leading biotechnology firm to co-develop a next-generation monoclonal antibody specifically targeting ITP. This collaboration is poised to enhance Amgen's research capabilities and accelerate the development timeline for new therapies, potentially positioning the company as a leader in the ITP space. The strategic importance of this partnership lies in its potential to leverage combined expertise, thereby increasing the likelihood of successful clinical outcomes and market acceptance.
In September 2025, Bristol-Myers Squibb (US) launched a clinical trial for a novel immunotherapy aimed at patients with refractory ITP. This initiative underscores the company's commitment to addressing unmet medical needs within the ITP population. The trial's outcomes could significantly influence treatment paradigms, particularly if the therapy demonstrates superior efficacy compared to existing options. Such advancements may not only enhance patient outcomes but also solidify Bristol-Myers Squibb's position as a frontrunner in the therapeutic landscape.
In October 2025, Novartis (CH) completed the acquisition of a smaller biotech firm specializing in gene therapy for hematological disorders, including ITP. This acquisition is strategically significant as it allows Novartis to diversify its portfolio and integrate cutting-edge gene therapy technologies into its existing treatment frameworks. The move reflects a broader trend within the industry towards innovative treatment modalities that promise to revolutionize patient care in ITP and similar conditions.
As of October 2025, the competitive trends within the ITP therapeutics market are increasingly defined by digitalization, sustainability, and the integration of artificial intelligence in drug development processes. Strategic alliances are becoming more prevalent, as companies recognize the value of collaboration in navigating complex regulatory environments and accelerating innovation. Looking ahead, it is likely that competitive differentiation will evolve from traditional price-based strategies to a focus on technological advancements, innovative therapies, and robust supply chain management. This shift suggests a future where companies that prioritize research and development, alongside strategic partnerships, will be best positioned to thrive in the ITP therapeutics market.
















Leave a Comment