Rising Prevalence of IBS
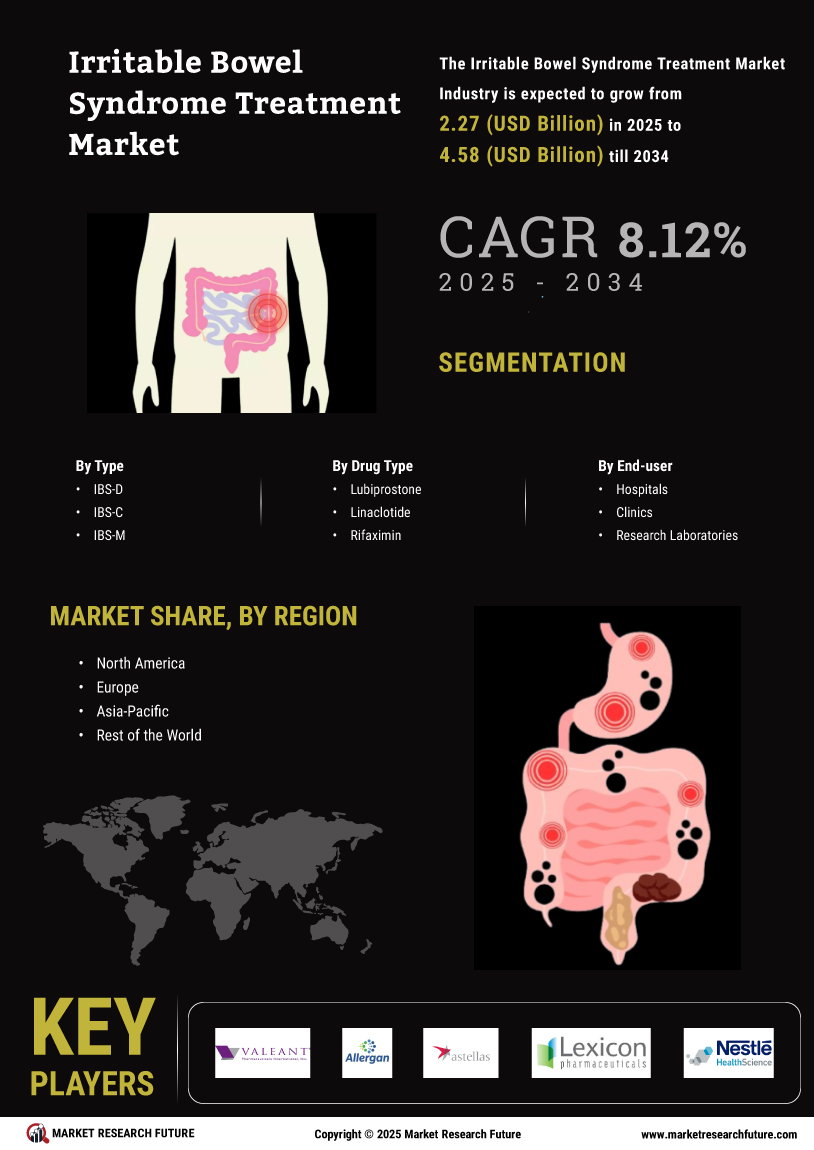
The increasing prevalence of Irritable Bowel Syndrome (IBS) globally is a primary driver for the Global Irritable Bowel Syndrome Treatment Market Industry. It is estimated that around 10 to 15% of the global population suffers from IBS, leading to a heightened demand for effective treatment options. This growing patient population is expected to contribute significantly to the market, with the industry projected to reach 2.1 USD Billion in 2024. As awareness of IBS continues to rise, healthcare providers are more likely to seek innovative therapies, further propelling market growth.
Rising Healthcare Expenditure
An increase in global healthcare expenditure is facilitating growth in the Global Irritable Bowel Syndrome Treatment Market Industry. As countries allocate more resources to healthcare, patients gain better access to diagnostic tools and treatment options for IBS. This trend is particularly evident in developed nations, where healthcare systems are increasingly prioritizing gastrointestinal disorders. The financial commitment to healthcare is likely to enhance the availability of IBS treatments, thus supporting market growth as more patients seek effective management solutions.
Advancements in Treatment Options
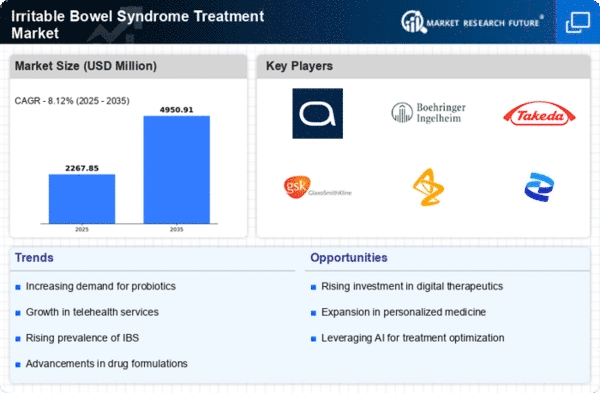
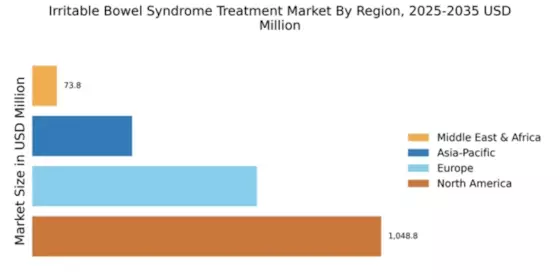
Innovations in treatment methodologies are shaping the Global Irritable Bowel Syndrome Treatment Market Industry. Recent developments in pharmacological therapies, including the introduction of new medications specifically targeting IBS symptoms, are enhancing patient outcomes. For instance, the approval of novel agents that address both constipation and diarrhea subtypes of IBS has expanded the therapeutic landscape. This evolution in treatment options is likely to attract more patients seeking relief, thereby driving the market's growth trajectory towards an anticipated valuation of 4.95 USD Billion by 2035.
Increased Awareness and Diagnosis
The growing awareness surrounding IBS and its symptoms is significantly influencing the Global Irritable Bowel Syndrome Treatment Market Industry. Enhanced educational initiatives by healthcare organizations are leading to improved diagnosis rates. As more individuals recognize their symptoms and seek medical advice, the demand for IBS treatments is expected to rise. This trend is likely to contribute to a compound annual growth rate (CAGR) of 8.11% from 2025 to 2035, reflecting a robust market expansion as more patients are diagnosed and treated effectively.
Growing Interest in Personalized Medicine
The shift towards personalized medicine is transforming the Global Irritable Bowel Syndrome Treatment Market Industry. Tailored treatment approaches that consider individual patient profiles are becoming more prevalent, leading to improved therapeutic outcomes. This trend is driven by advancements in genetic research and a deeper understanding of IBS pathophysiology. As healthcare providers adopt personalized strategies, the market is expected to benefit from increased patient satisfaction and adherence to treatment regimens, ultimately contributing to the overall growth of the industry.