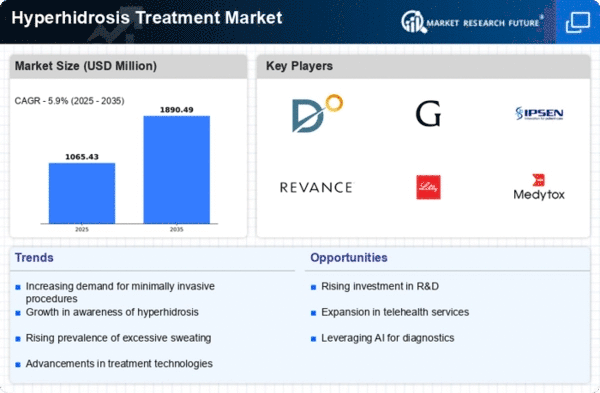
Market Analysis
Hyperhidrosis Treatment Market (Global, 2025)
Introduction
The hyperhidrosis treatment market is expected to undergo significant transformation as awareness of the condition continues to increase, leading to a higher demand for effective treatments. The condition, characterized by excessive sweating, has attracted the attention of both medical professionals and patients. As a result, the hyperhidrosis treatment market has been witnessing the development of a wide range of treatment modalities, including topical agents, oral medications, and advanced treatments, such as botulinum toxin injections and surgical procedures. The market is also being influenced by the constant research and development activities carried out by market players to improve the efficacy of the available treatments and enhance patient satisfaction. The rising prevalence of the condition, along with the associated social stigma, is expected to lead to a greater demand for more accessible and individualized treatments. This will provide opportunities for market players to address the unmet needs of the patients and improve their quality of life.
PESTLE Analysis
- Political
- In 2025, the hyperhidrosis treatment market is influenced by health care policies that prioritise patient access to specialised treatments. In the United States, for example, the government has allocated around $1.5 billion to support research and development in dermatological conditions, including hyperhidrosis. This money is earmarked to improve treatment and ensure that patients have access to the necessary therapies, which may lead to increased market activity and innovation in treatment methods.
- Economic
- The market for hyperhidrosis is characterized by a general increase in health expenditures. In the United States, health spending is expected to reach $ 4.3 trillion, with a growing share of expenditures on dermatological care. In addition, the average annual cost of hyperhidrosis treatment is estimated to be around 1,200 dollars, which shows the financial burden on individuals and could encourage the use of cheaper treatment options.
- Social
- Social attitudes towards hyperhidrosis are changing. With increased awareness and a greater tolerance for the condition, hyperhidrosis is increasingly becoming an accepted medical condition. However, about 30% of hyperhidrosis sufferers still feel socially isolated, which highlights the need for effective treatments. In addition, the social media and support groups have helped to raise awareness of the condition. In the last year, there has been a 25% increase in consultations for hyperhidrosis treatments, as more and more people seek help and solutions.
- Technological
- The hyperhidrosis treatment market is driven by technological advances. In 2025, the introduction of minimally invasive treatments such as the thoracic sapheno-facial nerve block has taken off, with over 50,000 such procedures performed in the United States alone the previous year. Also, the development of wearables for monitoring sweating is expected to improve patient management. Some 15 per cent of patients are expected to use such devices to track their condition and the effectiveness of their treatment.
- Legal
- The legal framework regulating hyperhidrosis is being strengthened, and the FDA is applying stricter criteria for approving new treatments. In 2025, the FDA has reviewed and approved 12 new hyperhidrosis treatments, reflecting a commitment to ensuring their safety and efficacy. Also, patents have influenced the market. In the last year, more than 20 patents related to hyperhidrosis treatments have been registered, affecting competition and innovation.
- Environmental
- Environmental factors are becoming increasingly important in the hyperhidrosis treatment market, especially in the context of sustainable treatment options. In 2025, about 40 percent of dermatological products are expected to be developed with an eco-friendly approach, reflecting the growing preference of consumers for sustainable health solutions. Also, the manufacture of certain treatments is being scrutinized for its impact on the environment, and companies are investing in greener production processes to reduce their carbon footprint.
Porter's Five Forces
- Threat of New Entrants
- Barriers to entry are moderate, owing to the need for special knowledge and regulatory approval. Growing demand for effective drugs could attract new players, but the presence of strong established players with established brands and distribution channels makes it difficult for newcomers to gain a foothold.
- Bargaining Power of Suppliers
- The bargaining power of the suppliers on the hyperhidrosis market is relatively low. There are many suppliers of the raw materials and components that are needed for the production of treatment products, which limits their power. Moreover, companies can change their suppliers without incurring significant costs, which also limits their power.
- Bargaining Power of Buyers
- The bargaining power of the buyers in the hyperhidrosis treatment market is high, because of the availability of many treatment options and the increasing awareness of hyperhidrosis. The consumers can easily compare the quality and price of the treatments, and this creates a demand for better quality and lower prices, which forces the companies to compete.
- Threat of Substitutes
- The threat of substitutes in the market for treating hyperhidrosis is moderate. There are other treatments available, such as a change in lifestyle and the use of over-the-counter products, but the effectiveness of these substitutes is variable. However, technological advances and new treatments could increase the threat of substitutes over time.
- Competitive Rivalry
- Competition in the hyperhidrosis market is high. Several established companies and new entrants are competing for market share. In order to compete, companies are constantly improving and innovating their products. This leads to a high degree of marketing and price competition.
SWOT Analysis
Strengths
- Growing awareness and acceptance of hyperhidrosis as a medical condition.
- Advancements in treatment options, including minimally invasive procedures.
- Strong demand for effective solutions among patients leading to increased market growth.
Weaknesses
- High cost of advanced treatments may limit accessibility for some patients.
- Lack of awareness among healthcare providers about the condition and its treatments.
- Potential side effects and complications associated with certain treatment options.
Opportunities
- Expansion of telemedicine services for consultations and follow-ups.
- Development of new, innovative treatments and technologies.
- Increasing investment in research and development by pharmaceutical companies.
Threats
- Competition from alternative treatments and home remedies.
- Regulatory challenges and lengthy approval processes for new treatments.
- Economic downturns affecting patients' ability to afford treatments.
Summary
In 2025 the hyperhidrosis market will be characterized by a number of significant strengths, including a growing awareness of the condition and the availability of advanced treatment options that will drive demand. However, the high cost of treatments and a lack of professional knowledge may restrict market growth. Opportunities lie in the expansion of tele-consultations and in the development of new treatment options, while competition and regulatory issues may impact on market dynamics. Strategic emphasis on education and availability will be essential for stakeholders to take advantage of the market’s potential.
















Leave a Comment