Market Analysis
In-depth Analysis of Host Cell Contaminant Testing Market Industry Landscape
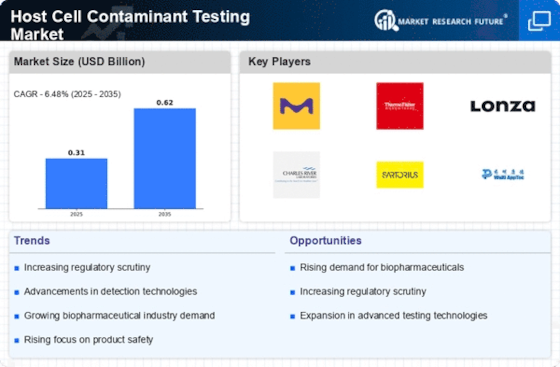
The host cell contaminants testing marketplace is an important aspect of the biopharmaceutical enterprise that specializes in detecting and tracking contaminants originating from host cells throughout the production of biologics. Market dynamics are fashioned by factors, which include regulatory requirements, technological improvements, and the increasing demand for safe and effective biopharmaceutical products. Stringent regulatory necessities pressure the demand for host cell contamination testing. Regulatory bodies mandate thorough checking out to ensure the safety and efficacy of biologics, shaping the marketplace dynamics and influencing testing methodologies. The growing manufacturing of biopharmaceuticals internationally is a key driver of the host cell contamination testing marketplace. As the biologics marketplace expands, the need for powerful testing strategies to identify and quantify Host Cell Contaminants rises in parallel. Ongoing improvements in testing technologies play a pivotal position in market dynamics. Improved detection strategies, which include mass spectrometry and polymerase chain reaction (PCR), decorate the sensitivity and specificity of host cell contaminants testing and meet the evolving needs of the biopharmaceutical enterprise. The globalization of biomanufacturing activities contributes to the market dynamics. As biopharmaceutical production extends to new areas, there may be a growing demand for host cell contamination testing offerings to make certain products the best and comply with worldwide requirements. Risk mitigation is a critical issue in the host cell contamination testing market. Robust testing methodologies help biopharmaceutical producers pick out and cope with ability risks associated with Host Cell Contaminants, ensuring product quality and patient safety. The dynamic nature of biopharmaceutical methods and checking out methods requires ongoing training and training. Companies invest in the continuous improvement of skilled specialists to ensure the proper execution of host cell contamination testing and adherence to evolving industry requirements. Effective statistics control and reporting are essential in host cell contamination testing. Companies with robust facts analysis and reporting talents are better placed to satisfy the growing demand for comprehensive documentation, facilitating regulatory compliance and patron delight. The absence of standardized testing protocols poses an undertaking in the host cell contamination testing market. Variability in trying out requirements across laboratories and areas may affect result interpretation and the comparability of information, emphasizing the want for standardization efforts. Similar to different industries, environmental sustainability is turning into attention in the host cell contamination testing marketplace. Companies are adopting environmentally pleasant practices in trying out procedures and disposal strategies to align with worldwide sustainability desires. Ongoing studies and development efforts cognizance of emerging technologies for Host Cell Contaminant testing. Innovations in testing methodologies and the identity of recent contaminants contribute to the continuous evolution of the marketplace.

















Leave a Comment