Market Analysis
HIV Diagnostics Market (Global, 2025)
Introduction
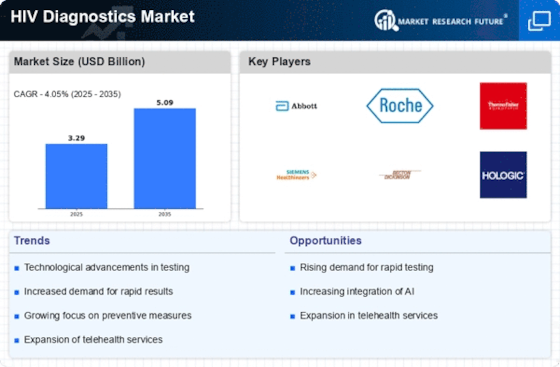
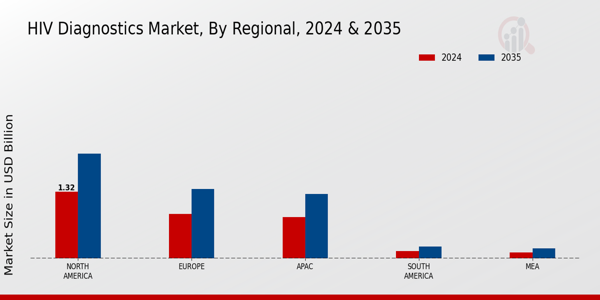
The HIV diagnostics market is undergoing significant change, as technological advancements and increased awareness of the threat of HIV and AIDS spur demand for new and better testing solutions. A growing focus on early detection and treatment has prompted the widespread adoption of rapid diagnostics and point-of-care testing. Further shaping the market is the integration of digital health, which makes remote monitoring and data management easier and improves patient outcomes. The rising prevalence of HIV in some regions also highlights the need for effective diagnostic tools, driving the public and private sectors to invest in research and development. The market will continue to evolve as it grapples with regulatory challenges and the need for cost-effective solutions. In the end, the market is likely to see a dynamic interplay between traditional and novel diagnostics, with the ultimate goal of reducing the global burden of HIV.
PESTLE Analysis
- Political
- The market for AIDS diagnostics in 2025 will be largely influenced by government policies designed to combat the AIDS epidemic. For example, the U.S. government has allocated approximately $1.1 billion to HIV prevention and treatment programs, which includes funding for diagnostics. The same is true for other developed countries. Moreover, international organizations, such as the Global Fund, have pledged $4.5 billion to support HIV/AIDS prevention and treatment in low- and middle-income countries, which highlights the importance of accessible diagnostics in these regions.
- Economic
- The HIV diagnostics market in 2025 will be affected by rising healthcare costs, which are projected to reach $4.3 trillion in the United States alone. This increase is partly due to the need for more advanced diagnostic tools to improve the treatment of patients. The cost of testing has fallen by up to 30% over the last five years, which makes it more affordable for both health care professionals and patients. In developing countries, the cost of testing is now around $10 per test.
- Social
- The social attitude towards the test and the treatment has changed a lot. It is now estimated that 70 per cent of the population in the high-prevalence areas is in favour of routine testing. It is the result of a marked increase in public information and education, which has led to a 25 per cent increase in the number of people tested each year. Also the stigma has been greatly reduced, and a recent survey shows that 60 per cent of the population believe that people living with HIV should have the same rights as people without the virus.
- Technological
- The market for diagnostics for AIDS is a major field of development. The introduction of rapid test kits which give results in less than 30 minutes has been particularly noteworthy. By 2025, it is estimated that over 50 million such kits will have been sold. This represents an increase of 40 per cent over the figure for 2024. In addition, the development of molecular diagnostics, such as PCR tests, have greatly improved the accuracy of the diagnosis. Moreover, the accuracy of the tests is over 95 per cent, which enables the detection of AIDS at an earlier stage and the initiation of treatment.
- Legal
- Legal frameworks for the diagnosis of AIDS are becoming more and more solid. Forty-five countries have enacted laws requiring the availability of a HIV test. The World Health Organization’s guidelines for 2025 require that at least ninety per cent of all people living with HIV know their status. This has resulted in a higher degree of surveillance and regulation in the field of diagnostics. Also, there are moves afoot to have the patent laws reviewed in order to make way for generic test-kits, which would lower the costs and increase access.
- Environmental
- The disposal of medical waste is a subject that is increasingly being addressed in the field of HIV diagnosis. By 2025 it is estimated that medical waste from hospitals will reach 16 million tons per year, a large part of which is due to diagnostics. In order to limit the impact on the environment, a large number of hospitals are implementing sustainable practices such as the proper disposal of medical waste and the implementation of a proper disposal procedure. In the coming years, the number of hospitals that have introduced a disposal procedure that is more friendly to the environment will rise to 30%.
Porter's Five Forces
- Threat of New Entrants
- The AIDS diagnostics market in 2025 is characterized by moderate barriers to entry. New players will have to overcome the technological and regulatory hurdles. But the growing demand for new diagnostics and technological innovations may encourage new entrants. The existing players have strong brand loyalty and distribution networks, which may discourage new entrants. However, the potential for high profits may also encourage some to enter the market.
- Bargaining Power of Suppliers
- Suppliers in the HI market generally have low bargaining power because of the availability of multiple sources for raw materials and components. There are many suppliers in the market, limiting their ability to influence prices. Also, many of the companies that make diagnostic products have the flexibility to change suppliers or even to produce components themselves, which reduces suppliers’ power even further.
- Bargaining Power of Buyers
- In the HIV diagnostics market, the buyers are the hospitals, clinics, and governmental agencies. The buyers have a high degree of bargaining power. The buyers can bargain for lower prices and better terms of payment, because of the increased focus on cost-effectiveness and the availability of more diagnostic choices. The buyers can also compare products, enhancing their bargaining power. This makes it essential for companies to offer both good prices and added-value services.
- Threat of Substitutes
- The threat of substitutes on the HIV diagnostics market is moderate. There are other testing methods and devices, such as home testing kits and rapid tests, but the sensitivity and specificity of the laboratory tests are still important. But as technology develops and new methods of testing are developed, the threat of substitutes may increase, forcing existing players to continuously improve.
- Competitive Rivalry
- Competition in the market for HIV diagnostics is high, with a large number of established players and a constant stream of new technology. Product differentiation and innovation are used to capture market share. Competition is further intensified by the need for continual improvement in diagnostic accuracy and speed.
SWOT Analysis
Strengths
- Advancements in technology leading to more accurate and faster diagnostic tests.
- Increased awareness and education about HIV leading to higher testing rates.
- Strong support from government and non-governmental organizations for HIV testing initiatives.
Weaknesses
- High costs associated with some advanced diagnostic technologies.
- Limited access to testing in rural and underserved areas.
- Stigma associated with HIV testing may deter individuals from seeking diagnosis.
Opportunities
- Growing demand for home-based and point-of-care testing solutions.
- Potential for partnerships with tech companies to enhance diagnostic capabilities.
- Expansion into emerging markets with rising HIV prevalence rates.
Threats
- Competition from alternative diagnostic methods and technologies.
- Regulatory challenges and changes in healthcare policies affecting market dynamics.
- Economic downturns that may limit funding for HIV testing programs.
Summary
The market for the diagnosis of AIDS in 2025 will be characterized by the strong growth of technological development and the growing awareness of the public. However, obstacles to access, such as high costs and stigma, will persist. Opportunities for growth will come from home testing and from alliances with technology companies. Threats from competition and regulatory changes will threaten the stability of the market. Strategic focus on removing obstacles and exploiting opportunities will be crucial for the participants in this developing market.

















Leave a Comment