Rising Prevalence of HIV
The increasing prevalence of HIV infections worldwide is a primary driver of the HIV Diagnostics Market. According to recent estimates, approximately 38 million people are living with HIV, with a significant number unaware of their status. This underscores the urgent need for effective diagnostic tools to identify and manage the disease. The rising number of new infections, particularly in regions with limited access to healthcare, further amplifies the demand for innovative diagnostic solutions. As awareness campaigns and testing initiatives expand, the market for HIV diagnostics is likely to experience substantial growth. The need for timely and accurate testing is critical in controlling the spread of HIV, thereby propelling the HIV Diagnostics Market forward.
Growing Awareness and Education
The growing awareness and education surrounding HIV/AIDS are pivotal in driving the HIV Diagnostics Market. Increased public knowledge about the importance of early detection and treatment has led to a rise in testing rates. Educational campaigns, often spearheaded by health organizations, aim to destigmatize HIV testing and encourage individuals to know their status. This shift in perception is crucial, as it fosters a culture of proactive health management. In recent years, the number of individuals seeking testing has surged, indicating a positive trend towards early diagnosis. As awareness continues to spread, the demand for HIV diagnostic tools is expected to rise, thereby enhancing the market landscape.
Emerging Markets and Accessibility
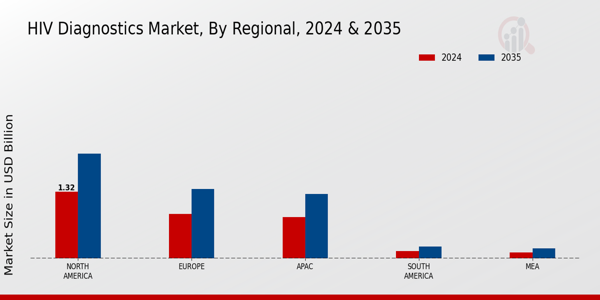
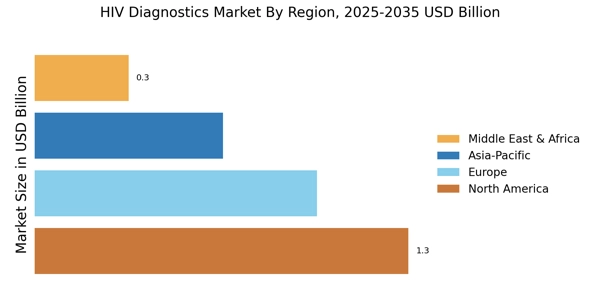
Emerging markets are becoming increasingly important in the HIV Diagnostics Market. As economies develop, there is a growing focus on improving healthcare infrastructure, which includes enhancing access to HIV testing. Countries in regions such as sub-Saharan Africa and parts of Asia are witnessing a surge in demand for affordable and effective diagnostic solutions. The expansion of healthcare services in these areas is likely to drive the adoption of innovative testing methods. Furthermore, partnerships between governments and private sectors are facilitating the distribution of diagnostic tools, making them more accessible to underserved populations. This trend suggests a promising future for the HIV Diagnostics Market as it adapts to meet the needs of diverse populations.
Government Initiatives and Funding
Government initiatives aimed at combating HIV/AIDS play a crucial role in shaping the HIV Diagnostics Market. Many countries have implemented national strategies to increase testing and treatment accessibility, often backed by substantial funding. For instance, public health campaigns and partnerships with non-governmental organizations have been established to promote awareness and facilitate testing. In 2023, funding for HIV-related programs reached billions of dollars, reflecting a commitment to reducing the incidence of HIV. Such investments not only enhance the availability of diagnostic tools but also encourage research and development in the field. As governments continue to prioritize HIV prevention and treatment, the market for diagnostics is expected to expand significantly.
Technological Innovations in Testing
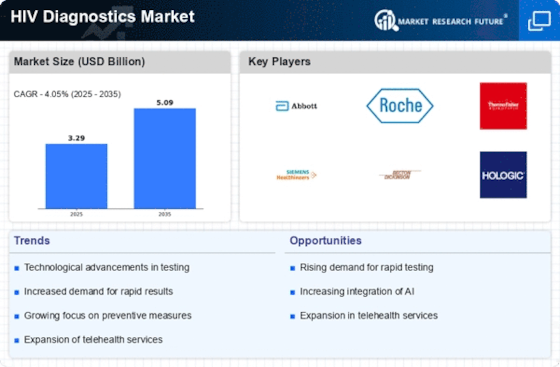
Technological advancements in diagnostic testing are transforming the HIV Diagnostics Market. Innovations such as rapid testing kits, molecular diagnostics, and point-of-care testing devices are enhancing the speed and accuracy of HIV detection. The introduction of these technologies has led to a notable increase in testing rates, as they provide results within minutes and can be used in various settings, including remote areas. The market for rapid HIV tests alone is projected to grow significantly, driven by the demand for user-friendly and efficient testing solutions. As technology continues to evolve, it is likely that new diagnostic methods will emerge, further propelling the growth of the HIV Diagnostics Market.