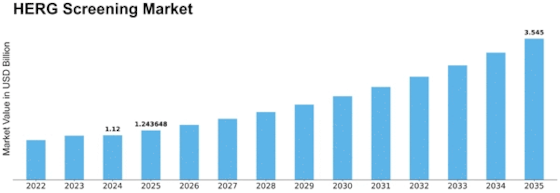
Herg Screening Size
HERG Screening Market Growth Projections and Opportunities
hERG screening is like a safety check for new medicines. When scientists are creating drugs, they need to make sure these drugs won't cause harm to the heart. hERG screening is a crucial part of this process. It checks if the drugs can block special channels in the heart called hERG potassium channels. These channels control how the heart's electricity works. If these channels are blocked, it can lead to a serious condition called long QT syndrome (LQTS). This condition can cause problems with the heart's rhythm and even lead to sudden cardiac death. So, hERG screening is like a protector, making sure new drugs won't cause harm to our hearts.
Now, the global hERG screening market is divided into different parts based on the type of channels it checks. The voltage-gated ion channel is one type, and it was the biggest in 2021. It's like the most common way to check these channels. Another type is the ligand-gated ion channel, and it's growing the fastest. It's like a newer and faster way to do the screening, making sure it's even better.
There are also different categories based on how the drugs are used. Some are for treating heart rhythm problems (antiarrhythmic), and this was the biggest category in 2021. Another category is for medicines that help with mental health (antipsychotic), and this is growing the fastest. It's like the use of these screenings is expanding to check different types of medicines, making sure they are all safe for our hearts.
In simple terms, hERG screening is like a superhero that checks if new medicines are safe for our hearts. The market for hERG screening is split into different parts based on the type of channels and the kind of medicines it checks. This helps scientists and doctors make sure that all sorts of medicines we use are safe and won't harm our hearts. As technology improves, these screenings get even better, making sure our hearts stay healthy when we take new medicines.


















Leave a Comment