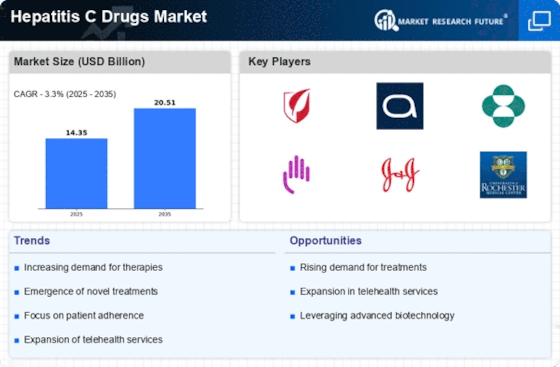
Market Analysis
In-depth Analysis of Hepatitis C Drugs Market Industry Landscape
The growing number of people infected with hepatitis C around the world has benefits for the global market. The widespread presence of the hepatitis C virus (HCV) is a big public health issue that drives the need for effective drugs and shapes market trends in the care and treatment of this viral disease.
The emergence of direct-acting antivirals (DAAs) has changed the market in a big way. Hepatitis C treatment has changed a lot since DAAs came out. They are very good at killing HCV at certain points in its lifecycle and don't cause side effects in most people. This has an effect on how competitive the market is and how well patients do.
More programs for screening and evaluation are good for the market. Trying to make people more aware of hepatitis C, encourage regular screening, and make testing tools better all help with early discovery and treatment. This changes market trends and lessens the long-term effects of hepatitis C.
The variety of HCV strains has a big effect on how markets work. Treatment responses vary depending on gene, which affects the creation of drugs that are special to genotypes and the choices that people make about their treatment plans.
Investigation of drug mixtures, such as multi-drug combos and fixed-dose combinations, leads to better treatment effectiveness and affects market trends in managing hepatitis C.
Hepatitis C activities around the world have an effect on how the market works. Targeting HCV with more tests, better care connections, and more people getting treatment has an effect on market growth and encourages healthcare organizations and drug companies to work together.
Patient access programs and attempts to make drugs more affordable have a big effect on how the market works. Pharmaceutical firms' efforts to make HCV drugs more available, especially in low- and middle-income countries, help the market grow and change how easy it is for people around the world to get these drugs.
On the market, there are a lot of methods for preventing harm and reducing it. Efforts to stop new HCV cases, especially in groups that are more likely to get them, change the way the market works by lowering the total disease load and determining the future demand for hepatitis C drugs.
The growing need for tracking and staging liver fibrosis shapes the size of the market. Keeping an eye on how liver fibrosis gets worse affects choices about treatment and the growth of non-invasive testing tools and liver health tests.
Problems with viruses becoming resistant to antivirals and treatments not working have a big effect on the market. Finding virus types that are immune and coming up with ways to deal with treatment fails are both parts of ongoing research that affects the strength of the market.
Researchers are paying close attention to the fact that HCV and HIV can co-infect people. The common co-infection of HCV and HIV leads to the creation of antiviral drugs that take dual therapy into account. This affects market decisions in drug research and treatment standards.

















Leave a Comment