Emergence of Telemedicine
The emergence of telemedicine is transforming the Hepatitis C Drugs Market by enhancing access to care for patients. Telehealth services allow individuals to consult healthcare providers remotely, facilitating timely diagnosis and treatment initiation without the need for in-person visits. This is particularly beneficial for patients in rural or underserved areas, where access to specialized care may be limited. As telemedicine becomes more integrated into healthcare systems, it is expected to increase patient engagement and adherence to treatment regimens. Moreover, the convenience of telehealth may encourage more individuals to seek testing and treatment for Hepatitis C, thereby driving demand within the Hepatitis C Drugs Market.
Growing Awareness and Education
Growing awareness and education regarding Hepatitis C are pivotal drivers for the Hepatitis C Drugs Market. Public health campaigns aimed at educating individuals about the risks, transmission, and treatment options for Hepatitis C have led to increased screening and diagnosis rates. As more people become aware of their Hepatitis C status, the demand for effective treatment options rises correspondingly. This heightened awareness is particularly important in high-risk populations, where stigma and misinformation have historically hindered access to care. Consequently, as educational initiatives continue to expand, the Hepatitis C Drugs Market is likely to experience sustained growth, driven by an informed patient population seeking treatment.
Advancements in Drug Development
Recent advancements in drug development are significantly influencing the Hepatitis C Drugs Market. The introduction of direct-acting antivirals (DAAs) has revolutionized treatment protocols, offering higher cure rates and shorter treatment durations compared to previous therapies. These innovations have not only improved patient outcomes but have also attracted substantial investments from pharmaceutical companies aiming to develop next-generation therapies. The market is witnessing a shift towards combination therapies that enhance efficacy and reduce the risk of resistance. As a result, the Hepatitis C Drugs Market is expected to expand, with new entrants and established players alike striving to capture market share through innovative product offerings.
Rising Prevalence of Hepatitis C
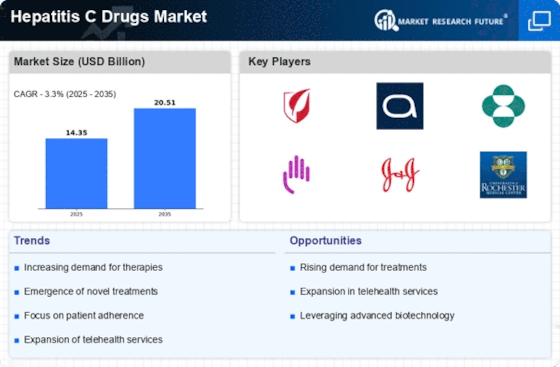
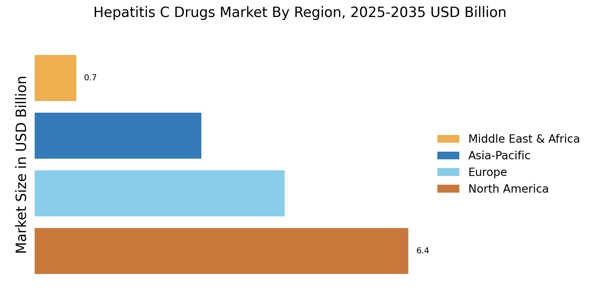
The increasing prevalence of Hepatitis C infections is a primary driver for the Hepatitis C Drugs Market. According to estimates, millions of individuals are living with chronic Hepatitis C worldwide, leading to a heightened demand for effective treatment options. This surge in cases is attributed to various factors, including changes in lifestyle, increased drug use, and lack of awareness regarding the disease. As the number of diagnosed patients rises, healthcare systems are compelled to invest in innovative therapies and medications, thereby propelling the Hepatitis C Drugs Market forward. Furthermore, the World Health Organization has set ambitious targets for eliminating Hepatitis C as a public health threat by 2030, which may further stimulate market growth as countries ramp up their treatment initiatives.
Government Initiatives and Funding
Government initiatives and funding play a crucial role in shaping the Hepatitis C Drugs Market. Many countries are implementing national strategies to combat Hepatitis C, which include increasing access to screening, diagnosis, and treatment. These initiatives often come with substantial financial backing, aimed at subsidizing the cost of medications and improving healthcare infrastructure. For instance, several nations have launched programs to provide free or low-cost access to DAAs, thereby increasing treatment uptake among affected populations. Such government-led efforts not only enhance public health outcomes but also stimulate market growth by creating a more favorable environment for pharmaceutical companies to operate within the Hepatitis C Drugs Market.