Stringent Regulatory Environment
The stringent regulatory environment in Germany significantly influences the pharmaceutical analytical-testing-outsourcing market. Regulatory bodies impose rigorous standards for drug testing and quality assurance, compelling pharmaceutical companies to adhere to strict compliance measures. This environment creates a demand for specialized analytical testing services that can navigate complex regulatory requirements. In 2025, it is anticipated that compliance-related expenditures will account for approximately 15% of total operational costs for pharmaceutical firms. Consequently, outsourcing analytical testing becomes a viable solution for companies aiming to meet these regulatory demands efficiently. The pharmaceutical analytical-testing-outsourcing market is thus likely to thrive as companies seek reliable partners to ensure compliance and maintain product quality.
Advancements in Analytical Technologies
Advancements in analytical technologies are playing a pivotal role in shaping the pharmaceutical analytical-testing-outsourcing market. Innovations such as high-throughput screening, mass spectrometry, and advanced chromatography techniques are enhancing the accuracy and efficiency of testing processes. These technological improvements enable pharmaceutical companies to obtain faster and more reliable results, which is crucial in the competitive landscape of drug development. In 2025, it is estimated that the adoption of these advanced technologies will increase by approximately 30% among outsourcing partners. This trend indicates a growing reliance on specialized analytical testing services that leverage cutting-edge technologies. Consequently, the pharmaceutical analytical-testing-outsourcing market is likely to expand as companies seek to capitalize on these advancements to improve their testing capabilities.
Growing Focus on Environmental Sustainability
The growing emphasis on environmental sustainability within the pharmaceutical sector is emerging as a significant driver for the analytical-testing-outsourcing market. Companies are increasingly adopting sustainable practices in their operations, including the testing phase of drug development. This shift is reflected in the rising demand for eco-friendly testing methods and materials. In 2025, it is projected that around 20% of pharmaceutical companies in Germany will prioritize sustainability in their testing processes. As a result, outsourcing partners that offer environmentally conscious testing solutions are likely to gain a competitive edge. The pharmaceutical analytical-testing-outsourcing market is expected to evolve in response to this trend, as companies seek to align their testing practices with sustainability goals.
Rising Demand for Outsourced Testing Services
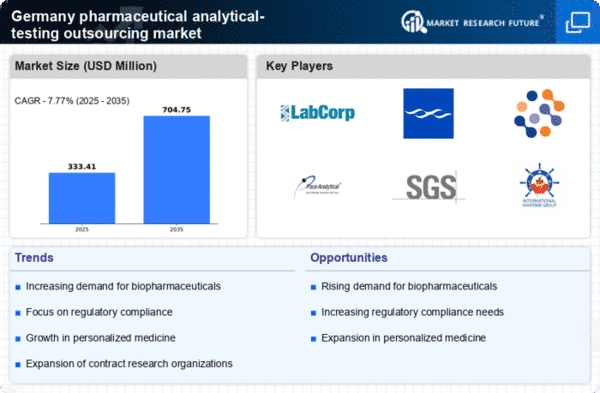
The pharmaceutical analytical-testing-outsourcing market in Germany is experiencing a notable increase in demand for outsourced testing services. This trend is driven by the need for pharmaceutical companies to enhance operational efficiency and reduce costs. By outsourcing analytical testing, companies can focus on core competencies while leveraging specialized expertise from service providers. In 2025, the market is projected to grow by approximately 8% annually, reflecting a shift towards outsourcing as a strategic approach. This growth is further supported by the increasing complexity of drug development processes, which necessitate advanced testing capabilities that outsourcing partners can provide. As a result, the pharmaceutical analytical-testing-outsourcing market is likely to expand significantly, driven by this rising demand for efficient and cost-effective testing solutions.
Increased Investment in Research and Development
Investment in research and development (R&D) within the pharmaceutical sector in Germany is a critical driver for the analytical-testing-outsourcing market. Pharmaceutical companies are allocating substantial budgets to R&D, with expenditures reaching over €10 billion in 2025. This investment is aimed at developing innovative therapies and improving existing products, which in turn necessitates comprehensive analytical testing. As companies seek to validate their R&D efforts, the reliance on outsourcing testing services is expected to grow. The pharmaceutical analytical-testing-outsourcing market is thus positioned to benefit from this trend, as R&D investments create a continuous demand for specialized testing services that ensure compliance with regulatory standards and support product development.