Aging Population
The demographic shift towards an aging population significantly influences the Global Genitourinary Drugs Market Industry. As individuals age, they become more susceptible to various genitourinary conditions, necessitating medical intervention. For instance, the World Health Organization reports that the global population aged 60 years and older is expected to double from 12% to 22% between 2015 and 2050. This demographic trend suggests an increasing demand for genitourinary drugs, as older adults often experience conditions such as urinary tract infections and prostate issues. Consequently, the market is likely to expand in response to this growing patient demographic.
Growing Awareness and Education
Enhanced awareness and education regarding genitourinary health are vital for the Global Genitourinary Drugs Market Industry. Public health campaigns and educational initiatives are increasingly informing patients about genitourinary disorders and available treatments. For instance, organizations are promoting discussions around urinary health, which helps reduce stigma and encourages individuals to seek medical advice. This growing awareness is likely to lead to earlier diagnosis and treatment, thereby increasing the demand for genitourinary drugs. As patients become more informed, the market is expected to benefit from a surge in treatment-seeking behavior.
Advancements in Drug Development
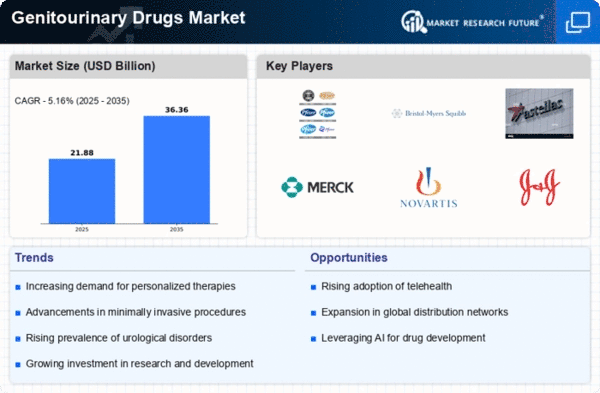
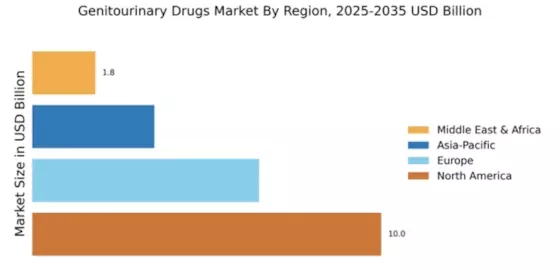
Innovations in drug development, including the introduction of novel therapeutics and targeted therapies, are pivotal for the Global Genitourinary Drugs Market Industry. Recent advancements in biotechnology have led to the creation of more effective medications with fewer side effects, enhancing patient compliance and outcomes. For example, the development of new formulations for existing drugs has shown promise in improving efficacy. As the industry continues to embrace cutting-edge research and development, the market is projected to grow at a CAGR of 3.6% from 2025 to 2035, potentially reaching 111.4 USD Billion by 2035.
Increased Healthcare Expenditure
Rising healthcare expenditure across the globe is a crucial driver for the Global Genitourinary Drugs Market Industry. Governments and private sectors are investing more in healthcare, leading to improved access to medications and treatments for genitourinary disorders. For example, countries are allocating larger portions of their GDP to healthcare, which enhances the availability of essential drugs. This trend is expected to stimulate market growth, as increased funding allows for better healthcare infrastructure and patient education. As a result, the demand for genitourinary drugs is likely to rise, contributing to the overall market expansion.
Rising Prevalence of Genitourinary Disorders
The increasing incidence of genitourinary disorders, such as urinary incontinence and benign prostatic hyperplasia, drives the Global Genitourinary Drugs Market Industry. For instance, studies indicate that urinary incontinence affects approximately 25 million adults in the United States alone, highlighting a significant patient population in need of effective treatments. This growing prevalence is expected to contribute to the market's expansion, with projections suggesting that the Global Genitourinary Drugs Market will reach 75.5 USD Billion in 2024. As awareness and diagnosis improve, the demand for innovative therapies is likely to rise, further propelling market growth.