Growing Awareness of Product Safety
There is a growing awareness of product safety among consumers and regulatory bodies in the GCC, which is driving the viral clearance market. As public health concerns rise, stakeholders are increasingly prioritizing the safety of biopharmaceutical products. This heightened awareness is prompting manufacturers to invest in robust viral clearance measures to mitigate risks associated with viral contamination. The viral clearance market is responding to this demand by developing more effective and reliable clearance methods. Furthermore, regulatory agencies are likely to impose stricter guidelines, compelling companies to enhance their viral clearance protocols, thereby fostering growth in the market.
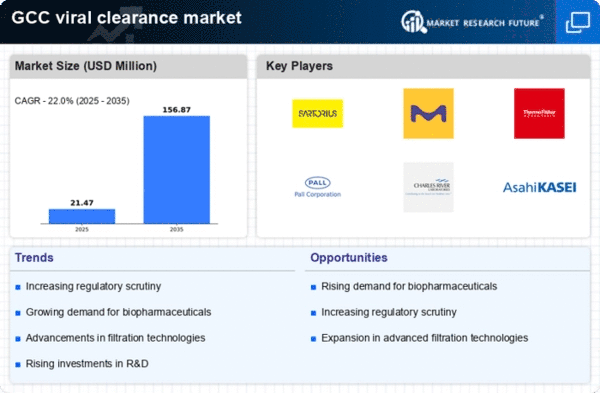
Rising Demand for Biopharmaceuticals
The increasing demand for biopharmaceuticals in the GCC region is a primary driver for the viral clearance market. As the biopharmaceutical sector expands, the need for effective viral clearance processes becomes critical to ensure product safety and efficacy. The GCC biopharmaceutical market is projected to grow at a CAGR of approximately 8% from 2025 to 2030, indicating a robust demand for viral clearance solutions. This growth is fueled by the rising prevalence of chronic diseases and the need for innovative therapies. Consequently, the viral clearance market is likely to experience heightened activity as manufacturers seek to comply with stringent safety standards and regulatory requirements, thereby enhancing their production capabilities.
Strategic Collaborations and Partnerships
Strategic collaborations and partnerships among key players in the GCC are emerging as a significant driver for the viral clearance market. Companies are increasingly joining forces to leverage each other's expertise and resources, facilitating the development of innovative viral clearance solutions. These collaborations often lead to the sharing of technology and knowledge, which can accelerate the introduction of new products into the market. The viral clearance market is likely to benefit from these partnerships as they enhance research and development efforts, ultimately leading to improved safety standards in biopharmaceutical manufacturing. This trend may also attract investment, further propelling market growth.
Emergence of Advanced Filtration Technologies
The emergence of advanced filtration technologies is reshaping the landscape of the viral clearance market. Innovations such as nanofiltration and ultrafiltration are gaining traction due to their effectiveness in removing viral contaminants from biopharmaceutical products. These technologies not only enhance the safety profile of products but also improve overall production efficiency. The market for filtration systems in the GCC is projected to grow significantly, with estimates suggesting a CAGR of around 10% over the next five years. As biopharmaceutical companies increasingly adopt these advanced filtration methods, the viral clearance market is likely to expand, driven by the need for more reliable and efficient viral clearance processes.
Increased Investment in Healthcare Infrastructure
The GCC countries are witnessing substantial investments in healthcare infrastructure, which significantly impacts the viral clearance market. Governments are allocating funds to enhance healthcare facilities, research institutions, and laboratories, thereby creating a conducive environment for the development of advanced viral clearance technologies. For instance, the healthcare expenditure in the GCC is expected to reach $100 billion by 2025, reflecting a commitment to improving healthcare services. This investment is likely to drive the demand for viral clearance solutions as healthcare providers and biopharmaceutical companies strive to meet the growing expectations for safety and quality in their products. The viral clearance market stands to benefit from this trend as new facilities emerge.