Growing Public Awareness
Public awareness regarding drug safety is on the rise in France, significantly impacting the pharmacovigilance market. Patients are becoming more informed about the potential risks associated with medications, leading to increased demand for transparency in drug safety reporting. This trend is prompting pharmaceutical companies to enhance their pharmacovigilance practices to maintain consumer trust. As a result, the market is projected to grow by approximately 6% over the next few years. The emphasis on patient-centric approaches and the need for effective communication strategies are likely to drive investments in pharmacovigilance systems. This growing awareness among the public is thus a crucial driver for the evolution of the pharmacovigilance market.
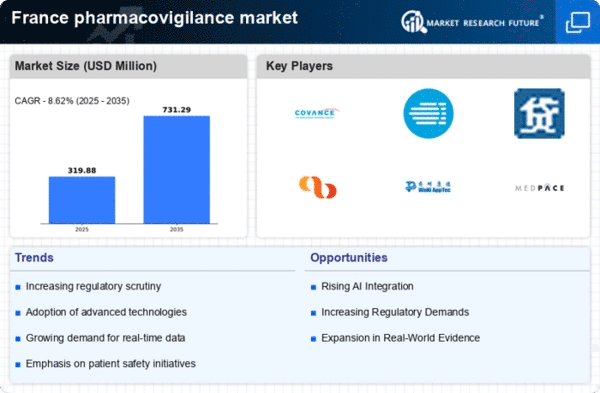
Increasing Regulatory Scrutiny
The pharmacovigilance market in France is experiencing heightened regulatory scrutiny, which is driving the demand for robust safety monitoring systems. Regulatory bodies, such as the French National Agency for Medicines and Health Products Safety (ANSM), are enforcing stricter guidelines for drug safety reporting. This has led to an increased need for companies to invest in comprehensive pharmacovigilance systems to ensure compliance. The market is projected to grow at a CAGR of approximately 8% over the next five years, as organizations strive to meet these evolving regulatory requirements. The emphasis on patient safety and risk management is likely to further propel the growth of the pharmacovigilance market in France, as stakeholders prioritize adherence to safety protocols.
Integration of Advanced Technologies
The integration of advanced technologies is transforming the pharmacovigilance market in France. The adoption of artificial intelligence (AI) and machine learning (ML) is enhancing data analysis capabilities, allowing for more efficient detection of adverse drug reactions. This technological evolution is expected to streamline reporting processes and improve the overall quality of safety data. As organizations increasingly leverage these technologies, the market is anticipated to witness a growth rate of around 7% annually. Furthermore, the use of big data analytics is enabling companies to analyze vast amounts of information, leading to more informed decision-making in drug safety. Consequently, the pharmacovigilance market is likely to expand as firms invest in these innovative solutions.
Rising Investment in Drug Development
The rising investment in drug development is a significant driver for the pharmacovigilance market in France. As pharmaceutical companies allocate more resources to research and development, the need for effective pharmacovigilance systems becomes increasingly critical. The market is projected to grow at a rate of approximately 6% annually, driven by the increasing complexity of drug formulations and the necessity for comprehensive safety monitoring. This trend is further supported by the growing number of clinical trials being conducted in France, which necessitates robust pharmacovigilance practices to ensure patient safety. Consequently, the investment in drug development is likely to bolster the pharmacovigilance market as companies seek to comply with regulatory requirements and safeguard public health.
Collaboration with Healthcare Providers
Collaboration between pharmaceutical companies and healthcare providers is becoming increasingly vital in the pharmacovigilance market. Such partnerships facilitate the sharing of safety data and enhance the reporting of adverse events. In France, healthcare professionals play a crucial role in identifying and reporting drug-related issues, which is essential for effective pharmacovigilance. This collaborative approach is expected to contribute to a market growth rate of around 5% in the coming years. By fostering stronger relationships with healthcare providers, pharmaceutical companies can improve their safety monitoring processes and ensure timely reporting of adverse drug reactions. This synergy is likely to enhance the overall effectiveness of the pharmacovigilance market.