Rising Awareness of Patient Safety
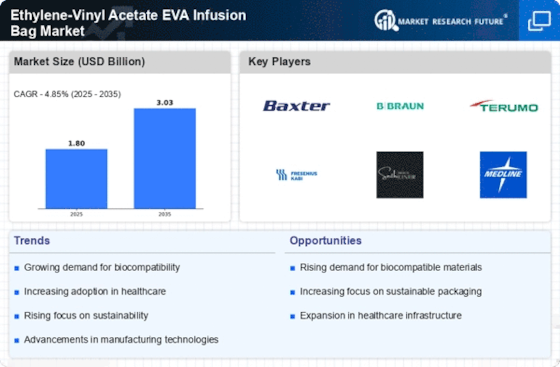
The Ethylene-Vinyl Acetate EVA Infusion Bag Market is experiencing a heightened awareness of patient safety, which is driving demand for safer and more effective infusion solutions. Healthcare providers are increasingly prioritizing the use of materials that minimize the risk of contamination and adverse reactions. Ethylene-Vinyl Acetate is recognized for its biocompatibility and safety profile, making it a preferred choice for infusion bags. This growing emphasis on patient safety is prompting manufacturers to invest in research and development to enhance the design and functionality of infusion bags. Additionally, educational initiatives aimed at healthcare professionals are likely to further promote the adoption of safer infusion practices. As a result, the Ethylene-Vinyl Acetate EVA Infusion Bag Market is poised for growth, as stakeholders recognize the importance of prioritizing patient safety in infusion therapies.
Shift Towards Home Healthcare Services
The Ethylene-Vinyl Acetate EVA Infusion Bag Market is witnessing a notable shift towards home healthcare services, driven by the increasing preference for at-home treatment options. Patients are increasingly opting for home-based therapies due to the convenience and comfort they offer. This trend is particularly evident among elderly patients and those with chronic conditions who require regular infusion therapies. As a result, healthcare providers are adapting their services to accommodate this demand, leading to an uptick in the use of Ethylene-Vinyl Acetate EVA infusion bags for home healthcare applications. The market for home healthcare is projected to grow significantly, with estimates suggesting a compound annual growth rate that could exceed 10% in the coming years. This shift not only enhances patient satisfaction but also reduces the burden on healthcare facilities, thereby driving the growth of the Ethylene-Vinyl Acetate EVA Infusion Bag Market.
Increasing Prevalence of Chronic Diseases
The Ethylene-Vinyl Acetate EVA Infusion Bag Market is significantly influenced by the rising prevalence of chronic diseases such as diabetes, cancer, and cardiovascular disorders. As these conditions require ongoing treatment and management, the demand for infusion therapies is expected to rise correspondingly. According to recent health statistics, the number of patients requiring intravenous therapies is projected to increase, thereby driving the need for efficient and reliable infusion bags. This trend is further compounded by an aging population that is more susceptible to chronic illnesses. Consequently, healthcare providers are increasingly relying on Ethylene-Vinyl Acetate EVA infusion bags to deliver medications and nutrients effectively. The growing patient population necessitates a robust supply chain for infusion products, positioning the Ethylene-Vinyl Acetate EVA Infusion Bag Market for substantial growth in the coming years.
Technological Innovations in Manufacturing
The Ethylene-Vinyl Acetate EVA Infusion Bag Market is experiencing a surge in technological innovations that enhance the manufacturing processes of infusion bags. Advanced techniques such as blow molding and extrusion are being adopted to improve the quality and efficiency of production. These innovations not only reduce manufacturing costs but also ensure that the bags meet stringent quality standards. As a result, manufacturers are able to produce lighter, more durable, and flexible infusion bags that cater to the evolving needs of healthcare providers. The integration of automation and smart technologies in production lines is likely to further streamline operations, thereby increasing output and reducing lead times. This trend appears to be a driving force in the Ethylene-Vinyl Acetate EVA Infusion Bag Market, as companies strive to maintain competitive advantages through enhanced product offerings.
Regulatory Compliance and Quality Assurance
The Ethylene-Vinyl Acetate EVA Infusion Bag Market is heavily influenced by the stringent regulatory compliance and quality assurance standards imposed by health authorities. Manufacturers are required to adhere to rigorous guidelines to ensure the safety and efficacy of infusion bags. This has led to increased investments in quality control measures and certifications, which are essential for market entry. Compliance with regulations such as ISO standards and FDA approvals is critical for manufacturers aiming to establish credibility and trust in the market. As healthcare providers prioritize patient safety, the demand for high-quality, compliant infusion bags is likely to rise. This focus on regulatory adherence not only enhances product reliability but also fosters innovation within the Ethylene-Vinyl Acetate EVA Infusion Bag Market, as companies strive to meet evolving standards.