Regulatory Focus
Regulatory bodies are placing heightened emphasis on the EndoAVF Device Market, which is influencing market dynamics. Stricter regulations and guidelines are being established to ensure the safety and efficacy of endovascular devices. This regulatory focus is likely to lead to increased scrutiny during the approval process, which may initially slow down the introduction of new products. However, it also encourages manufacturers to invest in research and development to meet these standards. The result is a more robust and reliable product offering in the market. As compliance becomes a critical factor, companies that can navigate these regulatory landscapes effectively are expected to gain a competitive edge. This evolving regulatory environment is anticipated to shape the future of the EndoAVF Device Market significantly.
Growing Patient Population
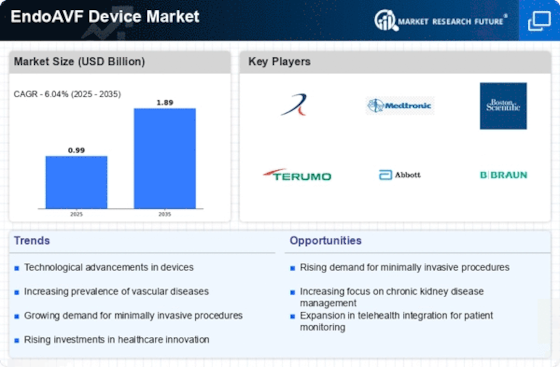
The EndoAVF Device Market is witnessing growth driven by an increasing patient population requiring vascular access solutions. The prevalence of chronic kidney disease and the rising number of patients undergoing dialysis are contributing to this trend. According to recent statistics, the number of patients requiring dialysis is expected to reach 5 million by 2026, creating a substantial demand for effective vascular access devices. This growing patient base is prompting healthcare providers to seek innovative solutions, thereby expanding the market for EndoAVF devices. Additionally, as awareness of the benefits of endovascular procedures increases, more patients are likely to opt for these minimally invasive options. Consequently, the EndoAVF Device Market is positioned for sustained growth as it adapts to the needs of an expanding patient demographic.
Technological Advancements
The EndoAVF Device Market is experiencing a surge in technological advancements that enhance the efficacy and safety of vascular access procedures. Innovations such as minimally invasive techniques and improved imaging technologies are transforming how endovascular arteriovenous fistulas are created. For instance, devices that utilize ultrasound guidance are becoming increasingly prevalent, allowing for more precise placements and reduced complications. The market is projected to grow at a compound annual growth rate (CAGR) of approximately 12% over the next five years, driven by these advancements. Furthermore, the integration of artificial intelligence in device design is likely to optimize patient outcomes, making procedures faster and more reliable. As healthcare providers seek to adopt the latest technologies, the EndoAVF Device Market is poised for significant growth.
Rising Awareness and Education
Rising awareness and education regarding vascular access options are significantly influencing the EndoAVF Device Market. Healthcare professionals and patients are becoming increasingly informed about the advantages of endovascular arteriovenous fistulas over traditional methods. Educational initiatives and training programs are being implemented to enhance understanding of these devices, leading to better patient outcomes. As awareness grows, more patients are likely to advocate for endoAVF procedures, thereby driving demand. Additionally, healthcare providers are recognizing the importance of educating their staff on the latest techniques and technologies, which is essential for successful implementation. This trend is expected to foster a more informed patient population, ultimately contributing to the expansion of the EndoAVF Device Market as it aligns with the evolving needs of both patients and healthcare providers.
Increased Investment in Healthcare
Investment in healthcare infrastructure is a pivotal driver for the EndoAVF Device Market. Governments and private entities are allocating substantial resources to enhance healthcare facilities and improve patient care. This trend is particularly evident in regions where healthcare systems are evolving to meet the demands of an aging population. Increased funding is facilitating the adoption of advanced medical technologies, including EndoAVF devices, which are essential for effective vascular access. As healthcare providers upgrade their facilities and equipment, the demand for innovative solutions is likely to rise. Furthermore, partnerships between technology firms and healthcare providers are becoming more common, fostering the development of cutting-edge devices. This influx of investment is expected to propel the EndoAVF Device Market forward, creating opportunities for growth and innovation.