Growing Regulatory Requirements
The Electronic Data Capture EDC System Market is increasingly shaped by stringent regulatory requirements governing clinical trials and data management. Regulatory bodies are placing greater emphasis on data integrity, security, and compliance, which necessitates the adoption of robust EDC systems. Organizations are compelled to invest in EDC solutions that not only meet these regulatory standards but also streamline the audit process. The market is expected to grow as companies seek to ensure compliance with evolving regulations, thereby minimizing the risk of penalties and enhancing their credibility in the eyes of stakeholders. This trend underscores the critical role of EDC systems in facilitating adherence to regulatory frameworks.
Rising Demand for Real-Time Data Access
The Electronic Data Capture EDC System Market is experiencing a notable surge in demand for real-time data access. This trend is largely driven by the need for timely decision-making in clinical trials and research studies. Organizations are increasingly recognizing that immediate access to data can enhance operational efficiency and improve patient outcomes. According to recent estimates, the market for EDC systems is projected to grow at a compound annual growth rate of approximately 12% over the next five years. This growth is indicative of the industry's shift towards more agile and responsive data management solutions, which are essential for meeting the evolving needs of stakeholders in the healthcare and research sectors.
Increased Focus on Patient-Centric Approaches
The Electronic Data Capture EDC System Market is witnessing a paradigm shift towards patient-centric approaches in clinical research. This shift emphasizes the importance of patient engagement and data collection methods that prioritize the patient experience. As a result, EDC systems are being designed to facilitate easier data entry for patients, thereby improving data quality and compliance. The market is expected to see a significant increase in the adoption of EDC solutions that incorporate mobile and remote data collection capabilities. This trend not only enhances patient participation but also aligns with regulatory expectations for patient involvement in research, potentially leading to more robust clinical outcomes.
Technological Advancements in Data Management
The Electronic Data Capture EDC System Market is significantly influenced by rapid technological advancements in data management. Innovations such as artificial intelligence and machine learning are being integrated into EDC systems, enabling more sophisticated data analysis and predictive modeling. These technologies allow for the automation of data entry and monitoring processes, which can reduce human error and enhance data integrity. As organizations seek to leverage these advancements, the market is projected to expand, with a focus on systems that offer enhanced functionalities and user-friendly interfaces. This evolution in technology is likely to redefine how data is captured, managed, and utilized in clinical research.
Expansion of Clinical Trials in Emerging Markets
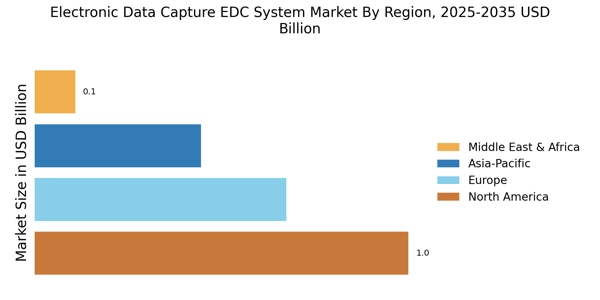
The Electronic Data Capture EDC System Market is benefiting from the expansion of clinical trials in emerging markets. As pharmaceutical companies and research organizations seek to tap into new patient populations, the demand for efficient data capture solutions is rising. Emerging markets offer diverse demographics and a growing pool of potential participants, making them attractive for clinical research. Consequently, EDC systems that can accommodate multi-site trials and diverse regulatory environments are becoming increasingly essential. This trend is likely to drive market growth as organizations aim to optimize their data collection processes and enhance the overall efficiency of clinical trials in these regions.