Market Share
Distraction Osteogenesis Devices Market Share Analysis
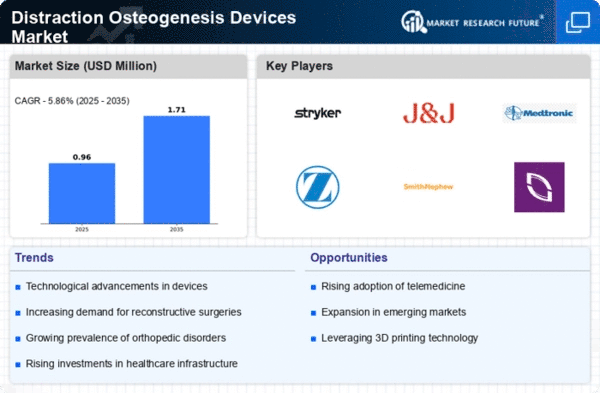
The Distraction Osteogenesis Devices market, a specialized segment within orthopedic and reconstructive surgery, involves devices used to gradually lengthen bones and promote tissue regeneration. Effective market share positioning in this niche market requires strategic approaches tailored to the unique needs of patients and healthcare professionals. Developing solid relationships with orthopedic surgeons as well as research institutions is an important approach. These joint ventures ensure that there is sharing of vital information relating to patient's needs, aid in the development of novel gadgets and create a platform for influential health-based experts. The health authorities' adherence to harsh regulatory standards and assessing the safety of distractive osteogenesis devices are highly important. Firms that follow the rules and keep safety first as their guiding principle form a great reputation which convince medical practitioners and consumers that they do the right thing. Education of patients and healthcare employees about the advantages and applicability of distraction osteofacial devices is inevitable. Organizations that invest in initiatives aimed at raising awareness and ensuring educational forums have a positive effect on the level of the adoption of their products by the market ultimately contributing to the market share. The ability to grow business internationally, tailored for different regional needs can bring about increment of market shares. By grasping the special needs of different national health care systems and patient populations, companies can personalize products to the exact ailments. Designing fairly cost-effective means for delivering distraction osteogenesis without accurately sacrificing the quality is a strategic approach. Allowing for selling devices which have a persuasive value proposition like the reduced treatment time, fewer complication and great outcome results sets the bar high for health care providers seeking compelling solutions. Comfort of the patient can be insured by tuning devices to his liking. Such customer centered approach may make companies outshine the competitors in the market. Devices that are minimally invasive, user-friendly, and comfortable to the patient during the distraction process could be the favorable helping other healthcare providers and the patient to be able to come up with the asking equipment.
Establishing robust post-market surveillance mechanisms and actively seeking user feedback contribute to ongoing device improvement. Companies that use real-world data and feedback to enhance their distraction osteogenesis devices demonstrate a commitment to continuous improvement and customer satisfaction.

















Leave a Comment