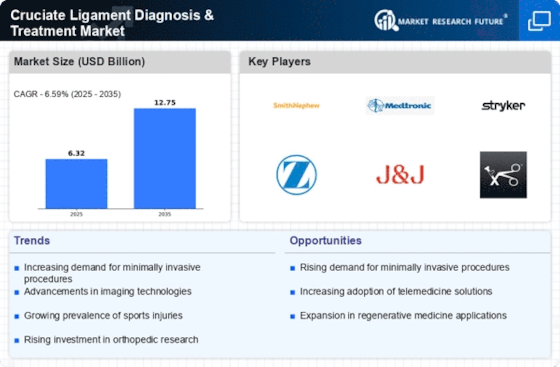
Top Industry Leaders in the Cruciate Ligament Diagnosis Treatment

Latest Cruciate Ligament Diagnosis and Treatment Companies Update
Jan 2023: The FDA has approved the ACL TightRope implant for pediatric patients, according to a recent announcement from Arthrex, a leader in lightweight surgical technologies. The first and only anterior cruciate ligament (ACL) fixation device approved for use in children is the TightRope implant, which is utilized in the surgical cure of orthopedic injuries. Alongside leading orthopedic surgeons, Arthrex developed all-epiphyseal and transphyseal procedures and instrumentation for ACL surgery. The FDA-approved additional indications and the addition of pediatric and young adolescent-specific hardware and implants to Arthrex's knee ligament array constitute a significant advance over current treatment choices for this high-risk group of athletes.
Jan 2023: Leading global provider of medical technology, Zimmer Biomet Holdings, Inc., declared that it has finalized an agreement to pay $155 million at closing and up to more than $120 million, contingent upon meeting future regulatory and commercial objectives over three years, for the acquisition of Embody, Inc., a privately held medical device business focused on soft tissue healing. In 2023, the purchase is expected to be marginally dilutive to adjusted profits per share and contribute positively to overall revenue growth. The acquisition includes Embody's whole line of collagen-based biointegrative solutions, such as the biointegrative implant TAPESTRY® for tendon recuperation and TAPESTRY® RC, which was among the first arthroscopic implant devices for rotator cuff repair to support healing in the most difficult orthopedic soft tissue injuries.List of Cruciate Ligament Diagnosis and Treatment Key companies in the market
- Stryker Corporation
- Arthrex, Inc.
- Zimmer Biomet
- DePuy Synthes, Inc.
- CONMED Corporation
- Smith & Nephew plc.
- DJO Global Inc.
- Breg, Inc.
- Bauerfeind AG