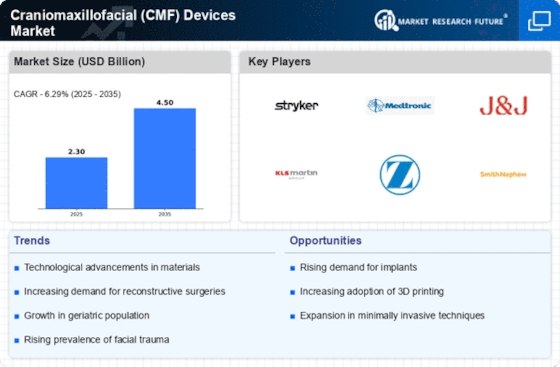
Rising Aesthetic Surgery Demand
The growing demand for aesthetic procedures is significantly influencing the Craniomaxillofacial (CMF) Devices Market. As societal perceptions of beauty evolve, more individuals are seeking surgical interventions to enhance facial features. This trend is particularly evident in regions where cosmetic surgery is becoming more socially accepted. Data indicates that the aesthetic segment of the CMF market is projected to witness substantial growth, driven by procedures such as rhinoplasty, chin augmentation, and facial implants. The increasing availability of minimally invasive techniques is likely to further propel this demand, as patients seek quicker recovery times and less invasive options. Consequently, manufacturers are focusing on developing innovative devices that cater to this burgeoning market.
Growing Incidence of Facial Trauma
The rising incidence of facial trauma, driven by factors such as road accidents, sports injuries, and interpersonal violence, is a critical driver for the Craniomaxillofacial (CMF) Devices Market. Statistics indicate that facial injuries account for a substantial percentage of trauma cases, necessitating effective surgical interventions. As the demand for reconstructive surgeries increases, the market for CMF devices is expected to expand correspondingly. The need for advanced fixation devices and implants to address complex fractures and deformities is becoming more pronounced. This trend suggests that healthcare providers are increasingly investing in innovative CMF solutions to improve patient outcomes and reduce the long-term impact of facial injuries.
Technological Innovations in CMF Devices
The Craniomaxillofacial (CMF) Devices Market is experiencing a surge in technological innovations that enhance surgical outcomes. Advanced materials, such as bioresorbable polymers and titanium alloys, are increasingly utilized in device manufacturing, improving biocompatibility and reducing the risk of complications. Furthermore, the integration of 3D printing technology allows for the customization of implants tailored to individual patient anatomies, which is likely to enhance surgical precision. According to recent data, the market for 3D-printed CMF devices is projected to grow significantly, reflecting a broader trend towards personalized medicine. These innovations not only improve patient satisfaction but also streamline surgical procedures, potentially reducing recovery times and hospital stays.
Aging Population and Associated Health Issues
The aging population presents a significant driver for the Craniomaxillofacial (CMF) Devices Market. As individuals age, they often experience a range of health issues, including bone density loss and facial deformities, which may necessitate surgical intervention. The demographic shift towards an older population is likely to increase the prevalence of conditions requiring CMF devices, such as osteoporotic fractures and tumors. Data suggests that the demand for reconstructive and corrective surgeries is expected to rise in tandem with this demographic trend. Consequently, manufacturers are focusing on developing devices that cater specifically to the needs of older patients, thereby enhancing their market presence and addressing the unique challenges posed by this demographic.
Increased Investment in Healthcare Infrastructure
The Craniomaxillofacial (CMF) Devices Market is benefiting from increased investment in healthcare infrastructure across various regions. Governments and private entities are recognizing the importance of advanced medical technologies in improving patient care. This investment is leading to the establishment of specialized surgical centers equipped with state-of-the-art CMF devices. Enhanced healthcare facilities are likely to facilitate more complex surgical procedures, thereby driving demand for CMF devices. Furthermore, as healthcare systems evolve, there is a growing emphasis on training healthcare professionals in the latest surgical techniques, which may further stimulate market growth. The overall improvement in healthcare infrastructure is expected to create a conducive environment for the expansion of the CMF devices market.