Market Trends
Key Emerging Trends in the Clinical Laboratory Test Market
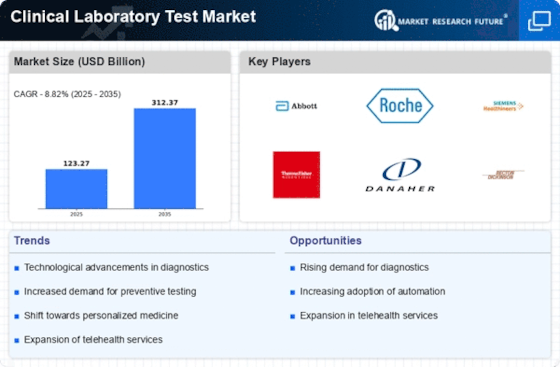
The clinical laboratory test market is experiencing significant growth and evolution, driven by various factors including technological advancements, demographic changes, and the increasing prevalence of chronic diseases. One key trend in this market is the growing demand for personalized and precision medicine, which requires a comprehensive range of laboratory tests to tailor treatment plans to individual patient characteristics and disease profiles. As our understanding of genetics, molecular biology, and biomarkers continues to expand, there is increasing interest in specialized tests such as genetic testing, molecular diagnostics, and companion diagnostics that enable more targeted and effective therapies for patients with cancer, genetic disorders, and other complex conditions.
Moreover, there is a shift towards automation and digitalization in clinical laboratories, driven by the need for increased efficiency, accuracy, and throughput. Automated laboratory systems, robotics, and laboratory information management systems (LIMS) are streamlining laboratory workflows, reducing turnaround times, and improving the reliability of test results. Additionally, digital pathology, remote monitoring, and cloud-based platforms are enabling seamless integration of laboratory data with electronic health records (EHRs) and facilitating collaboration among healthcare providers, researchers, and patients. These technological advancements are enhancing the quality of care, enabling more informed decision-making, and driving growth in the clinical laboratory test market.
Furthermore, there is increasing emphasis on point-of-care testing (POCT) and decentralized testing solutions to improve accessibility and convenience for patients, particularly in underserved or remote areas. POCT devices such as glucose meters, rapid diagnostic tests, and home testing kits enable patients to monitor their health status and manage chronic conditions more effectively outside of traditional healthcare settings. Additionally, decentralized testing platforms enable faster diagnosis and treatment initiation in emergency situations, critical care settings, and community health clinics, leading to improved patient outcomes and reduced healthcare costs. As technological advancements continue to miniaturize and simplify testing platforms, the market for POCT and decentralized testing is expected to expand further.
Another notable trend in the clinical laboratory test market is the increasing adoption of liquid biopsy and non-invasive testing methods for cancer detection and monitoring. Liquid biopsy techniques such as circulating tumor DNA (ctDNA) analysis, circulating tumor cells (CTCs) enumeration, and exosome analysis offer minimally invasive alternatives to traditional tissue biopsies for cancer diagnosis, prognosis, and treatment monitoring. These tests enable clinicians to detect tumor mutations, monitor treatment response, and identify resistance mechanisms more quickly and accurately, guiding personalized treatment decisions and improving patient outcomes. As liquid biopsy technologies continue to advance, they are becoming increasingly integrated into routine oncology practice, driving growth in the clinical laboratory test market.
Moreover, regulatory changes and healthcare policies are influencing the clinical laboratory test market, particularly in terms of quality assurance, reimbursement policies, and compliance requirements. Regulatory agencies are establishing standards for laboratory accreditation, proficiency testing, and test validation to ensure the accuracy and reliability of laboratory results. Additionally, healthcare payers are implementing value-based reimbursement models, bundled payment systems, and utilization management strategies to control costs and promote appropriate utilization of laboratory tests. Manufacturers and laboratory providers are adapting to these regulatory and reimbursement changes by investing in quality management systems, evidence-based testing guidelines, and cost-effective testing strategies to remain competitive in the evolving healthcare landscape.

















Leave a Comment