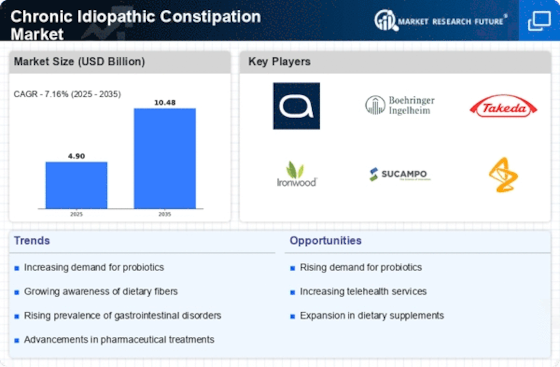
Top Industry Leaders in the Chronic Idiopathic Constipation Market

Latest Chronic Idiopathic Constipation Companies Updates:
Suven Therapeutics: Received FDA approval for SB207798, a first-in-class, peripherally acting G-protein coupled receptor 54 (GPR54) agonist, for treating CIC in adults.
Evoke Pharma: Launched Moviprep HCT, a new bowel preparation option for colonoscopy in CIC patients.
Iterum Therapeutics: Announced positive Phase 2b data for ITR-603, a novel, non-stimulant oral medication for treating CIC.
Almirall acquired Takeda's gastroenterology portfolio, including Movicol, a leading osmotic laxative used in CIC treatment.
Exelixis and Zai Lab entered into a collaboration to develop and commercialize Zai Lab's ET701, a potential first-in-class guanylate cyclase-C (GC-C) stimulator for treating CIC.
List of Chronic Idiopathic Constipation Key companies in the market:
- Pfizer Inc (U.S.)
- Progenics Pharmaceuticals (U.S.)
- GlaxoSmithKline (U.K.)
- Sucampo Pharmaceuticals Inc (U.S.)
- Ironwood Pharmaceuticals (U.S.)
- Sanofi (France)
- Salix Pharmaceuticals Ltd (U.S.)
- Bayer (Germany)