Government Initiatives and Funding
Government initiatives aimed at controlling sexually transmitted infections are pivotal in driving the Chlamydia Infection Diagnostics Market. Many countries have implemented national screening programs and awareness campaigns to combat the rising rates of chlamydia. These initiatives often come with substantial funding, which supports the development and distribution of diagnostic tools. For instance, public health organizations are increasingly investing in research and development to create more effective testing methods. This financial backing not only facilitates innovation but also ensures that diagnostic tools are accessible to a broader population. As governments continue to prioritize sexual health, the market is likely to experience sustained growth.
Shift Towards Preventive Healthcare
The transition towards preventive healthcare is a significant driver for the Chlamydia Infection Diagnostics Market. As healthcare systems worldwide increasingly prioritize prevention over treatment, there is a growing recognition of the importance of early detection of sexually transmitted infections. This shift encourages routine screenings and proactive health measures, which are essential for controlling the spread of chlamydia. The emphasis on preventive care is likely to result in higher demand for diagnostic tests, as healthcare providers seek to identify infections before they lead to more severe health complications. Consequently, this trend could foster a more dynamic market environment, with an array of innovative diagnostic solutions emerging to meet the needs of healthcare providers and patients alike.
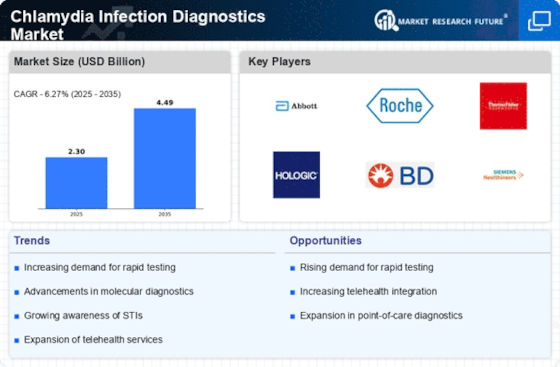
Rising Prevalence of Chlamydia Infections
The increasing incidence of chlamydia infections is a primary driver for the Chlamydia Infection Diagnostics Market. According to health statistics, chlamydia remains one of the most commonly reported sexually transmitted infections, particularly among young adults and adolescents. This rising prevalence necessitates effective diagnostic solutions to manage and control the spread of the infection. As awareness grows regarding the health implications of untreated chlamydia, healthcare providers are more inclined to adopt advanced diagnostic tools. The demand for accurate and rapid testing methods is likely to surge, thereby propelling the market forward. Furthermore, the need for routine screening in high-risk populations is becoming more recognized, which could further enhance the market landscape.
Increased Focus on Sexual Health Education
The growing emphasis on sexual health education is significantly influencing the Chlamydia Infection Diagnostics Market. Educational programs aimed at raising awareness about sexually transmitted infections, including chlamydia, are becoming more prevalent in schools and communities. This increased focus on education is likely to lead to higher rates of testing and diagnosis, as individuals become more informed about the risks and symptoms associated with chlamydia. As awareness campaigns gain momentum, the demand for reliable diagnostic solutions is expected to rise. Furthermore, educational initiatives often encourage regular screenings, particularly among high-risk groups, thereby creating a more robust market for diagnostic tools.
Technological Innovations in Diagnostic Tools
Technological advancements play a crucial role in shaping the Chlamydia Infection Diagnostics Market. Innovations such as nucleic acid amplification tests (NAATs) have revolutionized the diagnostic landscape, offering higher sensitivity and specificity compared to traditional methods. These advancements not only improve the accuracy of chlamydia detection but also reduce the time required for results, which is vital for effective patient management. The integration of point-of-care testing devices is also gaining traction, allowing for immediate diagnosis and treatment in various healthcare settings. As technology continues to evolve, the market is expected to witness the introduction of more sophisticated diagnostic solutions, which could significantly enhance testing capabilities and accessibility.