Stringent Regulatory Frameworks
The regulatory landscape in China is becoming increasingly stringent, particularly concerning the safety and efficacy of biopharmaceutical products. Regulatory bodies are enforcing rigorous guidelines that necessitate comprehensive viral clearance testing. This trend is likely to propel the growth of the viral clearance market, as companies must invest in advanced technologies to meet these requirements. In 2025, it is anticipated that compliance costs could account for up to 15% of total production expenses for biopharmaceutical manufacturers. Consequently, the demand for reliable viral clearance solutions is expected to rise, as companies strive to avoid costly penalties and ensure product approval. The evolving regulatory environment may also encourage innovation within the viral clearance market, as firms seek to develop more efficient and effective clearance methods.
Emergence of Advanced Technologies
The emergence of advanced technologies, such as nanotechnology and automation, is transforming the viral clearance market. In China, these innovations are enabling more efficient and effective viral clearance processes, which are essential for the biopharmaceutical industry. The integration of automation in manufacturing processes is expected to reduce operational costs by up to 20% by 2025, making it an attractive option for companies. The viral clearance market is likely to see increased adoption of these technologies as manufacturers seek to enhance productivity and ensure compliance with safety regulations. Furthermore, advancements in nanotechnology may lead to the development of novel viral clearance methods, potentially revolutionizing the industry. As these technologies continue to evolve, they could significantly impact the landscape of the viral clearance market in China.
Growing Awareness of Product Safety
There is a notable increase in consumer awareness regarding product safety in China, particularly concerning biopharmaceuticals. This heightened awareness is driving manufacturers to prioritize viral clearance processes to ensure the safety of their products. The viral clearance market is likely to benefit from this trend, as companies recognize the importance of maintaining high safety standards to build consumer trust. In 2025, it is estimated that approximately 70% of consumers will consider product safety as a critical factor in their purchasing decisions. As a result, manufacturers may invest more in viral clearance technologies to enhance their product offerings and meet consumer expectations. This shift towards prioritizing safety could lead to increased demand for innovative viral clearance solutions, further propelling market growth.
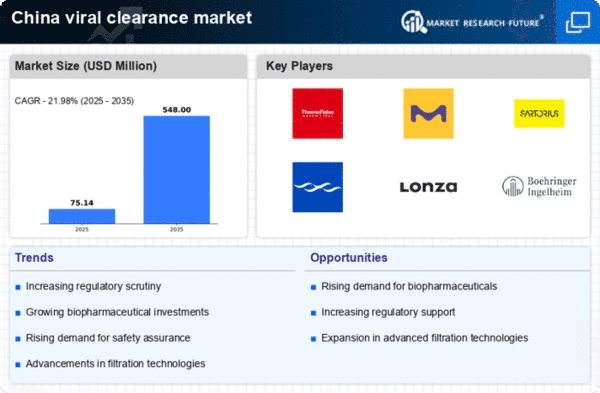
Rising Demand for Biopharmaceuticals
The increasing demand for biopharmaceuticals in China is a primary driver of the viral clearance market. As the biopharmaceutical sector expands, the need for effective viral clearance processes becomes critical to ensure product safety and efficacy. In 2025, the biopharmaceutical market in China is projected to reach approximately $100 billion, indicating a robust growth trajectory. This surge necessitates advanced viral clearance technologies to mitigate contamination risks. The viral clearance market is thus positioned to benefit from this trend, as manufacturers seek to comply with stringent safety regulations and maintain consumer trust. Furthermore, the growing prevalence of chronic diseases and the aging population in China are likely to further fuel the demand for biopharmaceuticals, thereby enhancing the need for reliable viral clearance solutions.
Increased Investment in Healthcare Infrastructure
China's ongoing investment in healthcare infrastructure significantly impacts the viral clearance market. The government has committed substantial resources to enhance healthcare facilities, which includes upgrading laboratories and manufacturing plants. This investment is expected to exceed $200 billion by 2025, creating a favorable environment for the adoption of advanced viral clearance technologies. As healthcare facilities modernize, the demand for efficient viral clearance processes is likely to rise, ensuring that biopharmaceutical products meet safety standards. The viral clearance market stands to gain from this trend, as companies seek to implement state-of-the-art technologies to comply with regulatory requirements and improve operational efficiency. Additionally, the expansion of healthcare infrastructure may lead to increased research and development activities, further driving the need for effective viral clearance solutions.