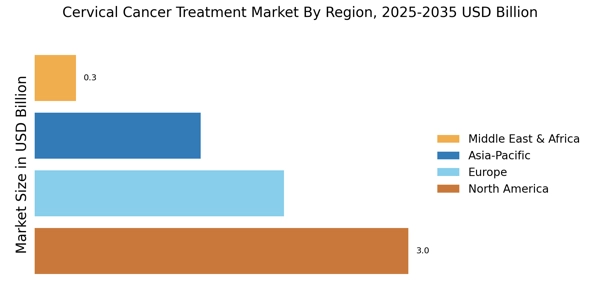Rising Awareness and Education
The growing awareness and education surrounding cervical cancer are vital drivers for the Cervical Cancer Treatment Market. Increased public knowledge about the disease, its risk factors, and the importance of early detection has led to higher screening rates. Educational campaigns, often supported by non-profit organizations and healthcare providers, are instrumental in informing women about preventive measures, including HPV vaccination. As awareness continues to rise, more women are likely to seek timely treatment, thereby increasing the demand for cervical cancer therapies. This trend is expected to create a more informed patient population, which could lead to improved treatment outcomes and further stimulate the Cervical Cancer Treatment Market.
Government Initiatives and Funding
Government initiatives aimed at combating cervical cancer are crucial drivers for the Cervical Cancer Treatment Market. Many countries are implementing national screening programs and vaccination campaigns to reduce the burden of cervical cancer. For example, funding for public health initiatives has increased, enabling better access to screening and treatment services. The allocation of resources towards research and development in cervical cancer therapies is also on the rise, as governments recognize the importance of addressing this health crisis. Such initiatives not only enhance awareness but also facilitate collaboration between public and private sectors, fostering innovation in treatment options. This supportive environment is likely to propel the growth of the Cervical Cancer Treatment Market.
Advancements in Treatment Technologies
Technological advancements in treatment modalities are significantly influencing the Cervical Cancer Treatment Market. Innovations such as targeted therapies, immunotherapies, and minimally invasive surgical techniques are transforming the landscape of cervical cancer treatment. For instance, the introduction of HPV vaccines has shown promise in reducing the incidence of cervical cancer, leading to a potential decrease in treatment needs. Furthermore, the development of personalized medicine approaches allows for tailored treatments based on individual patient profiles, enhancing efficacy and reducing side effects. As these technologies continue to evolve, they are expected to attract investment and drive market growth, making the Cervical Cancer Treatment Market more dynamic and responsive to patient needs.
Emerging Markets and Demographic Shifts
Emerging markets and demographic shifts are shaping the Cervical Cancer Treatment Market. As populations in developing regions grow and urbanization increases, the prevalence of cervical cancer is likely to rise. These regions often face challenges such as limited access to healthcare and lower screening rates, which can exacerbate the incidence of the disease. However, as economic conditions improve, there is potential for increased investment in healthcare infrastructure and treatment options. Additionally, the aging population in many countries is expected to contribute to a higher incidence of cervical cancer, further driving the demand for effective treatments. This demographic shift presents both challenges and opportunities for the Cervical Cancer Treatment Market.
Increasing Incidence of Cervical Cancer
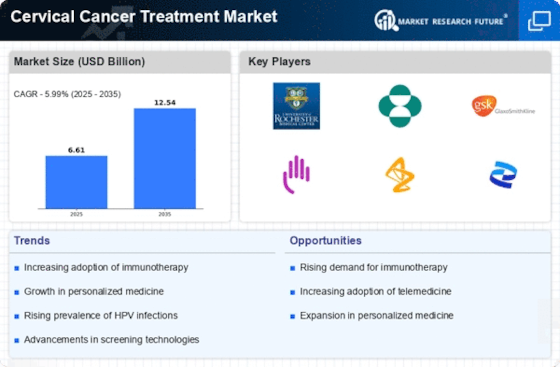
The rising incidence of cervical cancer is a pivotal driver for the Cervical Cancer Treatment Market. According to recent statistics, cervical cancer remains one of the leading causes of cancer-related deaths among women, particularly in developing regions. This alarming trend necessitates enhanced treatment options and increased healthcare investments. As awareness of cervical cancer grows, more women are seeking screening and treatment, thereby expanding the market. The World Health Organization has emphasized the need for comprehensive cervical cancer control programs, which further propels the demand for innovative therapies. Consequently, pharmaceutical companies are likely to invest in research and development to address this pressing health issue, thereby stimulating growth in the Cervical Cancer Treatment Market.