Emphasis on Infection Control
Infection control remains a paramount concern in healthcare, significantly influencing the Catheter Stabilization Market Devices Market. The rising awareness regarding hospital-acquired infections (HAIs) has led to increased scrutiny of catheter-related complications. Healthcare facilities are actively seeking solutions that minimize the risk of infections associated with catheter use. This has prompted manufacturers to innovate and produce devices that incorporate features aimed at enhancing sterility and reducing microbial colonization. The market is witnessing a shift towards products that not only stabilize catheters but also actively contribute to infection prevention. As a result, the demand for advanced catheter stabilization devices is expected to grow, with market analysts predicting a steady increase in sales as healthcare providers prioritize patient safety.
Growing Awareness and Education
There is a notable increase in awareness and education surrounding catheter management, which is positively impacting the Catheter Stabilization Market Devices Market. Healthcare professionals are becoming more informed about the importance of proper catheter stabilization techniques in preventing complications. Educational initiatives and training programs are being implemented to enhance the skills of healthcare providers in catheter care. This growing knowledge base is likely to lead to improved patient outcomes and a higher demand for effective catheter stabilization devices. As healthcare institutions prioritize training and education, the market for these devices is expected to expand, with an emphasis on products that align with best practices in catheter management.
Regulatory Support and Guidelines
The Catheter Stabilization Market Devices Market is benefiting from enhanced regulatory support and guidelines aimed at improving patient outcomes. Regulatory bodies are increasingly recognizing the importance of catheter stabilization in preventing complications associated with catheter use. This has led to the establishment of stringent guidelines that encourage the adoption of best practices in catheter management. Manufacturers are responding to these regulations by developing products that comply with safety standards and demonstrate efficacy. The alignment of industry practices with regulatory expectations is likely to foster innovation and drive market growth. As healthcare providers seek to adhere to these guidelines, the demand for compliant catheter stabilization devices is expected to rise, further propelling the market.
Rising Incidence of Chronic Diseases
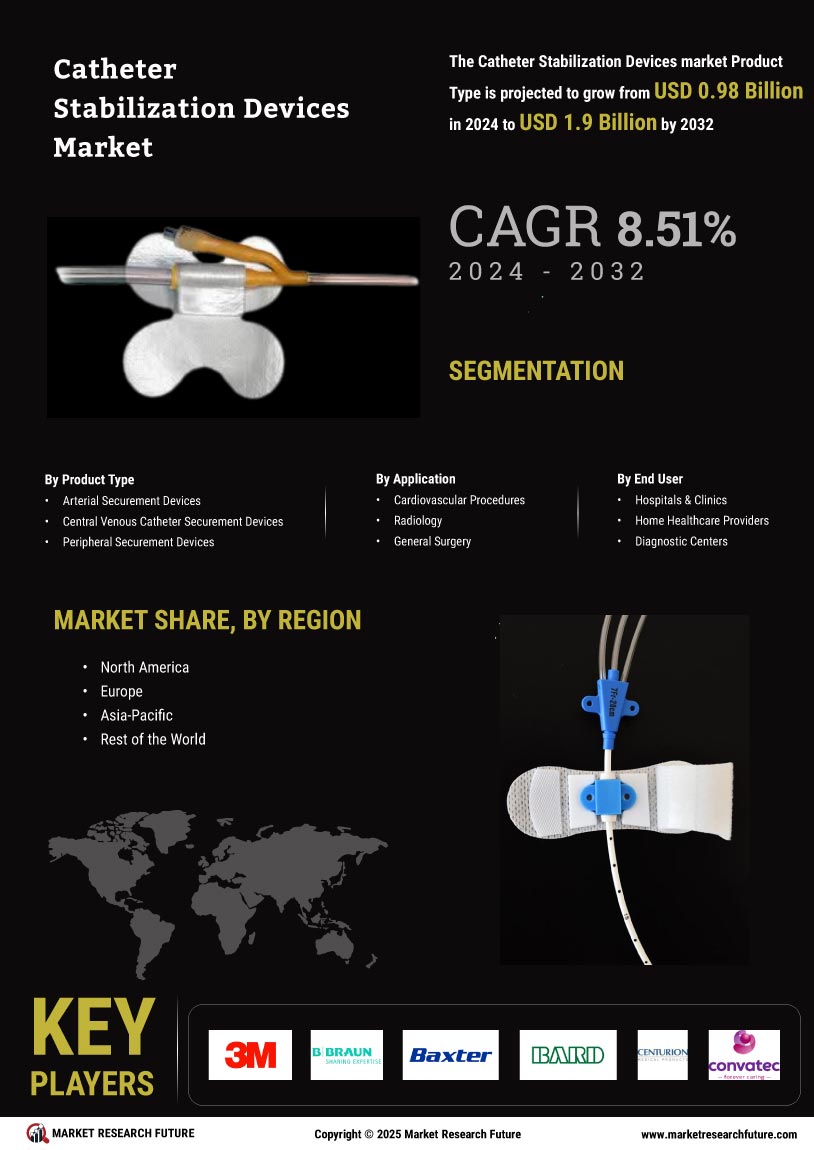
The increasing prevalence of chronic diseases is a significant driver for the Catheter Stabilization Market Devices Market. Conditions such as diabetes, cardiovascular diseases, and renal failure often necessitate the use of catheters for treatment and management. As the global population ages, the demand for effective catheterization solutions is likely to rise. According to recent statistics, the number of patients requiring long-term catheterization is projected to increase by approximately 15% over the next decade. This growing patient population underscores the need for reliable catheter stabilization devices that ensure safety and efficacy during treatment. Consequently, manufacturers are focusing on developing innovative solutions to meet this rising demand, thereby propelling market expansion.
Technological Innovations in Catheter Stabilization Devices
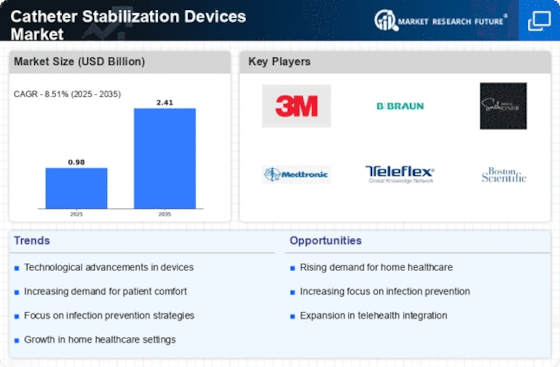
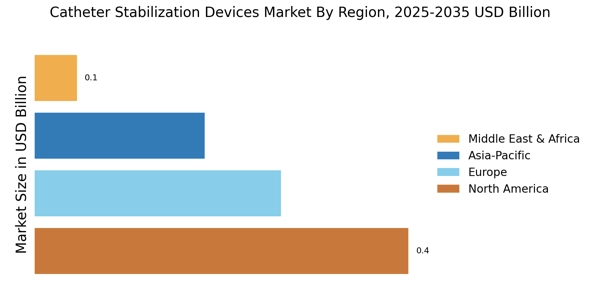
The Catheter Stabilization Market Devices Market is experiencing a surge in technological innovations that enhance the functionality and effectiveness of these devices. Advanced materials and designs are being developed to improve patient comfort and reduce the risk of complications. For instance, the integration of antimicrobial coatings in catheter stabilization devices has shown promise in minimizing infection rates, which is a critical concern in healthcare settings. Furthermore, the introduction of smart technology, such as sensors that monitor catheter position and alert healthcare providers to potential dislodgement, is likely to revolutionize patient care. As these innovations continue to emerge, they are expected to drive market growth, with projections indicating a compound annual growth rate of over 8% in the coming years.