Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
North America
Europe
South America
Asia Pacific
Middle East and Africa
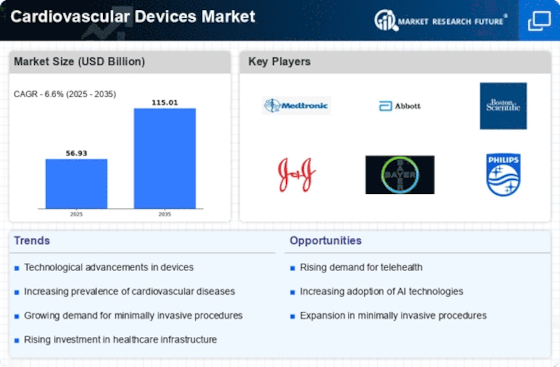
North America Outlook (USD Billion, 2019-2035)
North America Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
North America Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
North America Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
North America Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
North America Cardiovascular Devices Market by Regional Type
US
Canada
US Outlook (USD Billion, 2019-2035)
US Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
US Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
US Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
US Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
CANADA Outlook (USD Billion, 2019-2035)
CANADA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
CANADA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
CANADA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
CANADA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
Europe Outlook (USD Billion, 2019-2035)
Europe Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
Europe Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
Europe Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
Europe Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
Europe Cardiovascular Devices Market by Regional Type
Germany
UK
France
Russia
Italy
Spain
Rest of Europe
GERMANY Outlook (USD Billion, 2019-2035)
GERMANY Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
GERMANY Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
GERMANY Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
GERMANY Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
UK Outlook (USD Billion, 2019-2035)
UK Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
UK Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
UK Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
UK Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
FRANCE Outlook (USD Billion, 2019-2035)
FRANCE Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
FRANCE Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
FRANCE Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
FRANCE Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
RUSSIA Outlook (USD Billion, 2019-2035)
RUSSIA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
RUSSIA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
RUSSIA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
RUSSIA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
ITALY Outlook (USD Billion, 2019-2035)
ITALY Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
ITALY Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
ITALY Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
ITALY Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
SPAIN Outlook (USD Billion, 2019-2035)
SPAIN Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
SPAIN Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
SPAIN Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
SPAIN Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
REST OF EUROPE Outlook (USD Billion, 2019-2035)
REST OF EUROPE Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
REST OF EUROPE Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
REST OF EUROPE Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
REST OF EUROPE Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
APAC Outlook (USD Billion, 2019-2035)
APAC Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
APAC Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
APAC Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
APAC Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
APAC Cardiovascular Devices Market by Regional Type
China
India
Japan
South Korea
Malaysia
Thailand
Indonesia
Rest of APAC
CHINA Outlook (USD Billion, 2019-2035)
CHINA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
CHINA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
CHINA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
CHINA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
INDIA Outlook (USD Billion, 2019-2035)
INDIA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
INDIA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
INDIA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
INDIA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
JAPAN Outlook (USD Billion, 2019-2035)
JAPAN Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
JAPAN Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
JAPAN Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
JAPAN Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
SOUTH KOREA Outlook (USD Billion, 2019-2035)
SOUTH KOREA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
SOUTH KOREA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
SOUTH KOREA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
SOUTH KOREA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
MALAYSIA Outlook (USD Billion, 2019-2035)
MALAYSIA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
MALAYSIA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
MALAYSIA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
MALAYSIA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
THAILAND Outlook (USD Billion, 2019-2035)
THAILAND Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
THAILAND Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
THAILAND Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
THAILAND Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
INDONESIA Outlook (USD Billion, 2019-2035)
INDONESIA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
INDONESIA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
INDONESIA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
INDONESIA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
REST OF APAC Outlook (USD Billion, 2019-2035)
REST OF APAC Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
REST OF APAC Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
REST OF APAC Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
REST OF APAC Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
South America Outlook (USD Billion, 2019-2035)
South America Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
South America Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
South America Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
South America Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
South America Cardiovascular Devices Market by Regional Type
Brazil
Mexico
Argentina
Rest of South America
BRAZIL Outlook (USD Billion, 2019-2035)
BRAZIL Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
BRAZIL Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
BRAZIL Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
BRAZIL Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
MEXICO Outlook (USD Billion, 2019-2035)
MEXICO Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
MEXICO Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
MEXICO Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
MEXICO Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
ARGENTINA Outlook (USD Billion, 2019-2035)
ARGENTINA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
ARGENTINA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
ARGENTINA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
ARGENTINA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
REST OF SOUTH AMERICA Outlook (USD Billion, 2019-2035)
REST OF SOUTH AMERICA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
REST OF SOUTH AMERICA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
REST OF SOUTH AMERICA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
REST OF SOUTH AMERICA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
MEA Outlook (USD Billion, 2019-2035)
MEA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
MEA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
MEA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
MEA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
MEA Cardiovascular Devices Market by Regional Type
GCC Countries
South Africa
Rest of MEA
GCC COUNTRIES Outlook (USD Billion, 2019-2035)
GCC COUNTRIES Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
GCC COUNTRIES Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
GCC COUNTRIES Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
GCC COUNTRIES Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
SOUTH AFRICA Outlook (USD Billion, 2019-2035)
SOUTH AFRICA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
SOUTH AFRICA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
SOUTH AFRICA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
SOUTH AFRICA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
REST OF MEA Outlook (USD Billion, 2019-2035)
REST OF MEA Cardiovascular Devices Market by Device Type
Diagnostic Devices
Monitoring Devices
Therapeutic Devices
Surgical Devices
REST OF MEA Cardiovascular Devices Market by Application Type
Coronary Artery Disease
Heart Failure
Arrhythmia
Valvular Heart Disease
REST OF MEA Cardiovascular Devices Market by End User Type
Hospitals
Cardiac Centers
Ambulatory Surgical Centers
Home Healthcare
REST OF MEA Cardiovascular Devices Market by Technology Type
Implantable Pacemakers
Cardiac Stents
Ablation Devices
Heart Valve Devices
















Leave a Comment