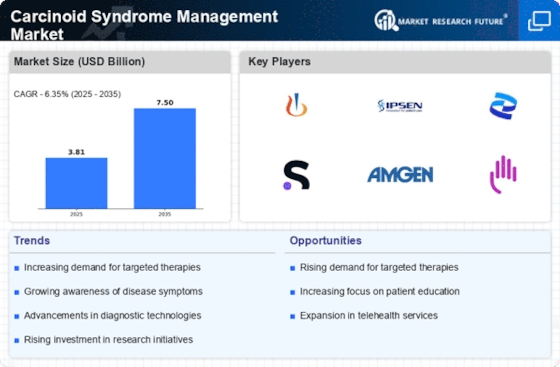
Top Industry Leaders in the Carcinoid Syndrome Management Market

Latest Carcinoid Syndrome Management Companies Updates:
TerSera Therapeutics Announces Positive Phase 3 Results for TS-130:In February 2023, TerSera announced favorable results from their Phase 3 trial for TS-130, their oral therapy for carcinoid syndrome diarrhea. This strengthens their position as a potential leader in this space.
Lexicon Pharmaceuticals Initiates Phase 3 Trial for LX211:Following positive Phase 2 results, Lexicon commenced a Phase 3 trial in January 2024 for LX211, another oral therapy targeting flushing symptoms. This signifies continued progress in developing convenient treatment options.
Ipsen and Sellas Collaboration Update:The partnership announced in October 2023 continues its progress, with successful expansion of Somatuline® Autogel access in several European countries. This improves availability of a well-established treatment for patients.
Carcinoid Syndrome Market Growth Projections Revised Upward:Recent market research reports like the one from Grand View Research (January 2024) suggest a faster-than-previously anticipated growth for the Carcinoid Syndrome Management Market, potentially reaching $6.2 billion by 2030. This reflects the increasing market interest and potential for promising treatments.
Focus on Patient Support and Advocacy:Organizations like the Carcinoid Cancer Foundation and the NET Patient Foundation are actively campaigning for increased awareness and improved access to resources and support for patients. This highlights the importance of holistic care beyond simply medications.
List of Carcinoid Syndrome Management Key companies in the market:
- Novartis International AG
- Pharmascience Inc. (Canada)
- Omega Laboratories Ltd. (Canada)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Mylan N.V. (USA)
- Ipsen Biopharmaceuticals, Inc.(US)
- Sirtex Medical Limited (Australia)