Market Growth Projections
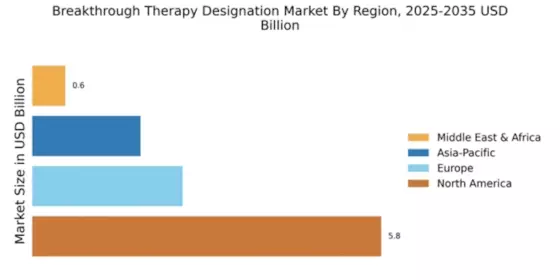
The Global Breakthrough Therapy Designation Market Industry is poised for substantial growth, with projections indicating a market value of 9.65 USD Billion in 2024 and an anticipated increase to 17.4 USD Billion by 2035. This growth reflects a compound annual growth rate of 5.48% from 2025 to 2035, driven by various factors such as regulatory support, increasing demand for innovative therapies, and advancements in biotechnology. The market's expansion is indicative of a broader trend towards prioritizing breakthrough therapies that offer significant improvements in treatment outcomes, thereby reshaping the pharmaceutical landscape.
Advancements in Biotechnology
Advancements in biotechnology play a crucial role in shaping the Global Breakthrough Therapy Designation Market Industry. Innovations in gene therapy, monoclonal antibodies, and personalized medicine are paving the way for new treatment modalities that can significantly alter disease trajectories. These advancements not only enhance the efficacy of therapies but also align with the criteria for breakthrough designation, which emphasizes the potential for substantial improvement over existing treatments. As biotechnology continues to evolve, it is anticipated that the market will expand, driven by the introduction of novel therapies that meet the rigorous standards set by regulatory bodies.
Regulatory Support and Incentives
Regulatory agencies worldwide are actively promoting the Global Breakthrough Therapy Designation Market Industry by providing incentives for the development of breakthrough therapies. These incentives include expedited review processes, reduced fees, and increased communication with regulatory authorities. Such support encourages pharmaceutical companies to invest in research and development, potentially leading to a greater number of therapies receiving breakthrough designation. This trend is expected to contribute to the market's growth, with projections indicating a value of 17.4 USD Billion by 2035, as more therapies are recognized for their innovative approaches.
Rising Prevalence of Chronic Diseases
The Global Breakthrough Therapy Designation Market Industry is significantly influenced by the rising prevalence of chronic diseases, such as cancer, diabetes, and autoimmune disorders. As these conditions become more widespread, there is an urgent need for effective treatments that can improve patient outcomes. Breakthrough therapies often address unmet medical needs, making them attractive options for healthcare providers and patients alike. This growing demand is likely to propel the market forward, with a projected compound annual growth rate of 5.48% from 2025 to 2035, indicating sustained interest in breakthrough designations.
Increasing Demand for Innovative Therapies
The Global Breakthrough Therapy Designation Market Industry experiences a surge in demand for innovative therapies, particularly in the treatment of rare and complex diseases. This demand is driven by the need for faster and more effective treatment options that traditional pathways may not provide. As of 2024, the market is valued at approximately 9.65 USD Billion, reflecting the growing recognition of breakthrough therapies' potential. Regulatory bodies are increasingly prioritizing these therapies, which could lead to a more streamlined approval process, thereby enhancing patient access to life-saving treatments.
Increased Investment in Research and Development
Investment in research and development is a key driver of the Global Breakthrough Therapy Designation Market Industry. Pharmaceutical companies are allocating substantial resources to explore innovative treatment options that can qualify for breakthrough designation. This focus on R&D is essential for addressing the growing demand for effective therapies, particularly in areas with high unmet medical needs. As companies strive to bring new therapies to market, the financial commitment to R&D is expected to yield significant returns, contributing to the market's growth trajectory and enhancing the overall landscape of breakthrough therapies.