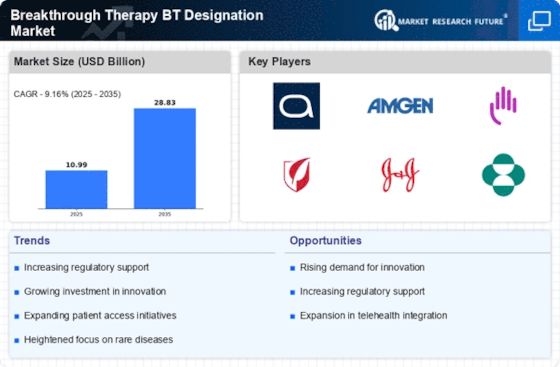
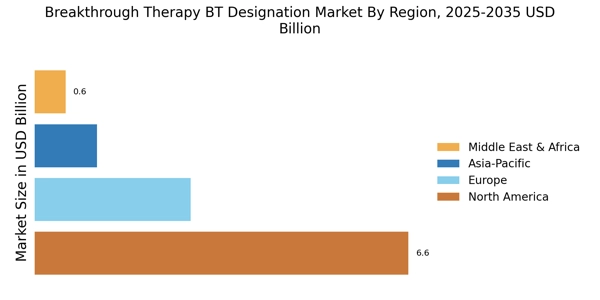
Increased Investment in R&D
The Breakthrough Therapy BT Designation Market is witnessing a surge in investment directed towards research and development. Pharmaceutical companies are increasingly allocating substantial resources to develop innovative therapies that meet the criteria for breakthrough designation. This trend is underscored by the fact that in recent years, the number of applications for breakthrough designation has risen significantly, indicating a robust pipeline of potential therapies. The financial commitment to R&D not only accelerates the development of new treatments but also enhances the competitive landscape within the industry. As companies strive to gain a foothold in the market, the influx of capital is likely to foster innovation and expedite the delivery of therapies to patients in need.
Patient Advocacy and Engagement
The Breakthrough Therapy BT Designation Market is significantly shaped by the growing emphasis on patient advocacy and engagement. Patients and advocacy groups are becoming increasingly vocal in their demands for faster access to innovative therapies. This shift in focus is prompting pharmaceutical companies to prioritize patient-centric approaches in their development strategies. The involvement of patients in clinical trial design and decision-making processes is becoming more common, which may lead to more relevant and effective therapies. As patient engagement continues to rise, it is likely to influence the direction of research and development within the industry, ensuring that therapies align more closely with patient needs and preferences.
Regulatory Flexibility and Support
The Breakthrough Therapy BT Designation Market benefits from an evolving regulatory environment that appears increasingly supportive of expedited drug development. Regulatory agencies have implemented frameworks that facilitate faster review processes for therapies designated as breakthrough. This flexibility is evidenced by the reduction in average review times, which has been reported to decrease by up to 50% for breakthrough therapies compared to standard applications. Such regulatory support not only encourages pharmaceutical companies to pursue breakthrough designations but also enhances the likelihood of successful market entry for innovative treatments. The proactive stance of regulatory bodies is likely to continue shaping the landscape of the industry, fostering a culture of rapid innovation.
Growing Demand for Rare Disease Treatments
The Breakthrough Therapy BT Designation Market is significantly influenced by the increasing demand for treatments targeting rare diseases. As awareness of rare conditions rises, there is a corresponding push for therapies that can address unmet medical needs. The market for rare disease treatments has expanded, with estimates suggesting that over 7,000 rare diseases affect millions of individuals worldwide. This growing demand creates a fertile ground for the development of breakthrough therapies, as companies seek to capitalize on the potential for high returns in niche markets. The intersection of patient advocacy and regulatory support is likely to further propel the development of innovative therapies within this segment.
Technological Advancements in Drug Development
The Breakthrough Therapy BT Designation Market is experiencing transformative changes driven by technological advancements in drug development. Innovations such as artificial intelligence and machine learning are being increasingly integrated into the research process, enabling more efficient identification of potential breakthrough therapies. These technologies facilitate the analysis of vast datasets, allowing for more precise targeting of drug candidates. As a result, the time required to bring a therapy from concept to market is likely to decrease, enhancing the overall efficiency of the development pipeline. The adoption of these technologies not only streamlines the process but also increases the likelihood of successful outcomes, thereby shaping the future of the industry.