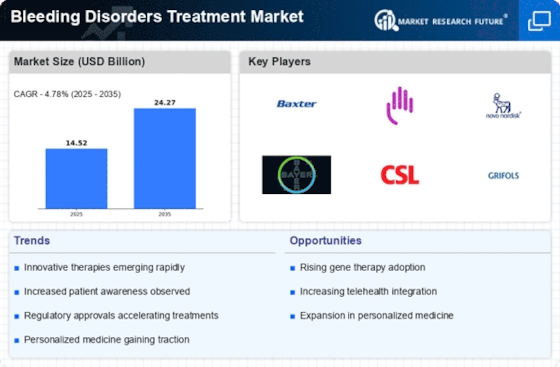
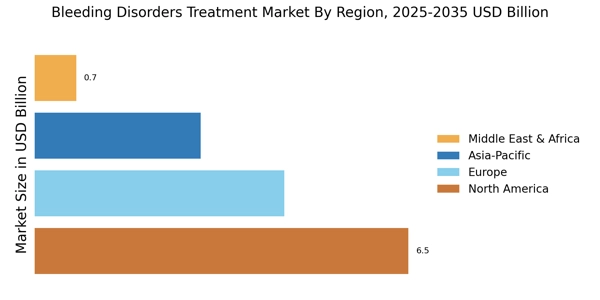
Advancements in Treatment Technologies
Technological innovations in the treatment of bleeding disorders are significantly influencing the Bleeding Disorders Treatment Market. The development of extended half-life factor concentrates and non-factor therapies has transformed the management of hemophilia, offering patients more convenient dosing regimens and improved quality of life. For instance, the introduction of subcutaneous therapies has the potential to simplify treatment administration, making it more accessible for patients. Additionally, the integration of digital health technologies, such as mobile applications for treatment tracking, is enhancing patient engagement and adherence. These advancements not only improve clinical outcomes but also stimulate market growth by attracting investments in research and development.
Rising Demand for Personalized Medicine
The shift towards personalized medicine is emerging as a crucial driver in the Bleeding Disorders Treatment Market. Tailoring treatment approaches based on individual patient profiles, including genetic factors and specific bleeding disorders, is becoming increasingly feasible. This trend is supported by advancements in genetic testing and biomarker identification, which allow for more precise treatment strategies. As a result, therapies can be optimized for efficacy and safety, potentially leading to better patient outcomes. The market is witnessing a growing interest in gene therapies that aim to provide long-term solutions for patients, thereby expanding the treatment landscape and driving market growth.
Growing Awareness and Education Initiatives
The increasing awareness and education surrounding bleeding disorders are significantly impacting the Bleeding Disorders Treatment Market. Various organizations and advocacy groups are actively promoting awareness campaigns to educate patients, healthcare professionals, and the general public about these conditions. This heightened awareness is leading to earlier diagnosis and treatment, which is crucial for effective management. Furthermore, educational initiatives are fostering a better understanding of available treatment options, thereby empowering patients to make informed decisions about their care. As awareness continues to grow, the demand for effective treatments is expected to rise, driving the overall market forward.
Increasing Prevalence of Bleeding Disorders
The rising incidence of bleeding disorders, such as hemophilia and von Willebrand disease, is a pivotal driver for the Bleeding Disorders Treatment Market. Recent estimates indicate that hemophilia affects approximately 1 in 5,000 male births, leading to a growing patient population requiring effective treatment options. This increasing prevalence necessitates advancements in therapeutic solutions, thereby propelling market growth. Furthermore, the awareness surrounding these disorders has improved, leading to earlier diagnoses and treatment initiation. As more individuals are identified and treated, the demand for innovative therapies, including factor replacement therapies and novel agents, is likely to surge, thereby enhancing the overall market landscape.
Regulatory Support and Market Access Initiatives
Regulatory frameworks and initiatives aimed at improving market access are playing a vital role in the Bleeding Disorders Treatment Market. Governments and health authorities are increasingly recognizing the need for effective treatments for bleeding disorders, leading to expedited approval processes for new therapies. For example, the implementation of orphan drug designations and fast-track approvals has facilitated quicker access to innovative treatments for patients. Additionally, reimbursement policies are evolving to support the inclusion of advanced therapies in treatment regimens, thereby enhancing patient access. This supportive regulatory environment is likely to encourage pharmaceutical companies to invest in research and development, further propelling market growth.