Regulatory Support and Funding
The Bladder Cancer Detection Kit Market is experiencing favorable regulatory support and increased funding, which are critical drivers for market growth. Governments and health organizations are recognizing the need for effective bladder cancer detection methods, leading to the establishment of supportive policies and funding opportunities for research and development. This regulatory environment encourages innovation and the introduction of new detection kits into the market. Furthermore, grants and subsidies aimed at improving cancer diagnostics are becoming more prevalent, providing financial backing for companies developing advanced detection technologies. As a result, the market is likely to see a proliferation of novel products designed to enhance early detection and improve patient outcomes. Analysts suggest that this trend could lead to a market expansion of over 15% in the next few years, highlighting the positive impact of regulatory frameworks on the Bladder Cancer Detection Kit Market.
Rising Incidence of Bladder Cancer
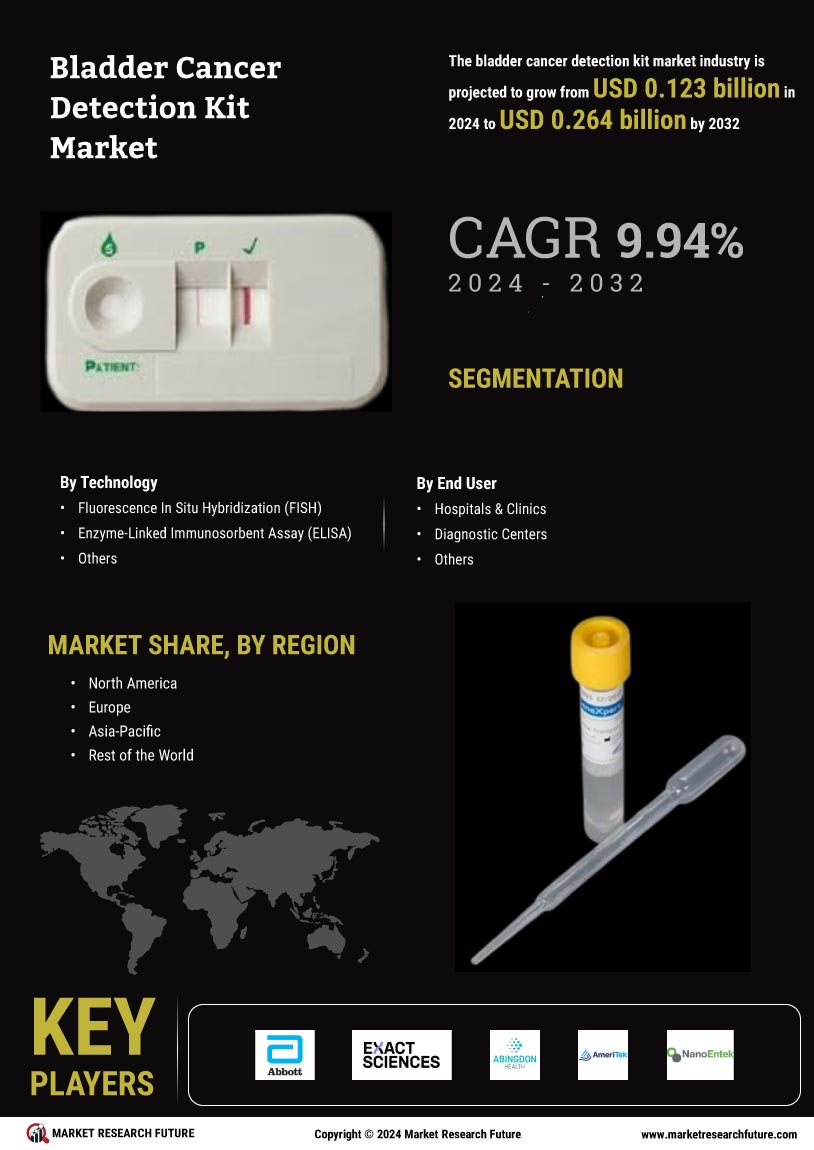
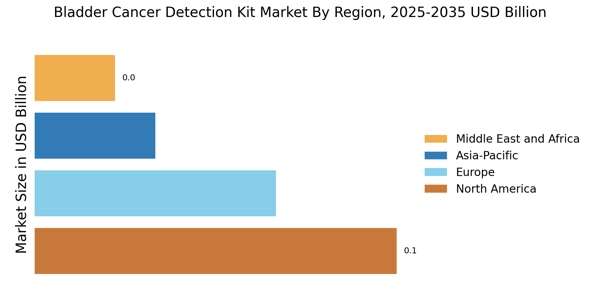
The Bladder Cancer Detection Kit Market is significantly influenced by the rising incidence of bladder cancer worldwide. Epidemiological studies indicate that bladder cancer is becoming increasingly prevalent, particularly among older adults and certain demographic groups. This trend is prompting healthcare systems to prioritize early detection and effective management strategies. As the number of diagnosed cases rises, the demand for reliable detection kits is expected to increase correspondingly. Market projections suggest that the bladder cancer detection kit segment could witness a growth rate of around 9% annually, driven by the urgent need for effective diagnostic solutions. This rising incidence not only underscores the importance of the Bladder Cancer Detection Kit Market but also highlights the critical role that early detection plays in improving survival rates and treatment outcomes.
Growing Investment in Cancer Research
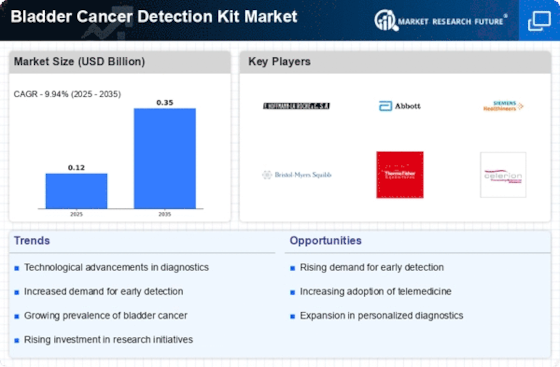
The Bladder Cancer Detection Kit Market is poised for growth due to the increasing investment in cancer research and development. As funding for cancer-related studies rises, there is a corresponding focus on developing innovative detection methods for various types of cancer, including bladder cancer. Research institutions and private companies are collaborating to create advanced diagnostic tools that can detect bladder cancer at earlier stages. This influx of investment is likely to accelerate the pace of innovation within the market, leading to the introduction of more effective detection kits. Analysts predict that this trend could result in a market growth rate of approximately 12% over the next few years, as new technologies emerge and existing products are refined. The commitment to cancer research is thus a pivotal factor driving the evolution of the Bladder Cancer Detection Kit Market.
Technological Advancements in Diagnostics
The Bladder Cancer Detection Kit Market is experiencing a surge in technological advancements that enhance diagnostic accuracy and efficiency. Innovations such as liquid biopsy and molecular imaging are revolutionizing how bladder cancer is detected. These technologies allow for non-invasive testing, which is increasingly preferred by patients and healthcare providers alike. The integration of artificial intelligence in diagnostic tools is also noteworthy, as it aids in the interpretation of complex data, potentially leading to earlier detection of bladder cancer. According to recent estimates, the market for bladder cancer detection technologies is projected to grow at a compound annual growth rate of over 10% in the coming years, driven by these advancements. As a result, the Bladder Cancer Detection Kit Market is likely to witness a significant transformation, improving patient outcomes and reducing healthcare costs.
Increased Awareness and Screening Initiatives
The Bladder Cancer Detection Kit Market is benefiting from heightened awareness regarding bladder cancer and the importance of early detection. Public health campaigns and educational initiatives are playing a crucial role in informing individuals about risk factors and symptoms associated with bladder cancer. This increased awareness is leading to a rise in screening initiatives, which are essential for early diagnosis and treatment. For instance, organizations are advocating for routine screenings among high-risk populations, such as smokers and individuals with a family history of bladder cancer. As a result, the demand for bladder cancer detection kits is expected to rise, with market analysts projecting a growth rate of approximately 8% annually. This trend underscores the importance of proactive health measures and the potential for the Bladder Cancer Detection Kit Market to expand significantly in response to these initiatives.